Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with it 1. An initial rate experiment was performed to determine the rate law of: 1BrO31+5Br1+6H+3Br2+3H2O The data that was collected is as
please help with it 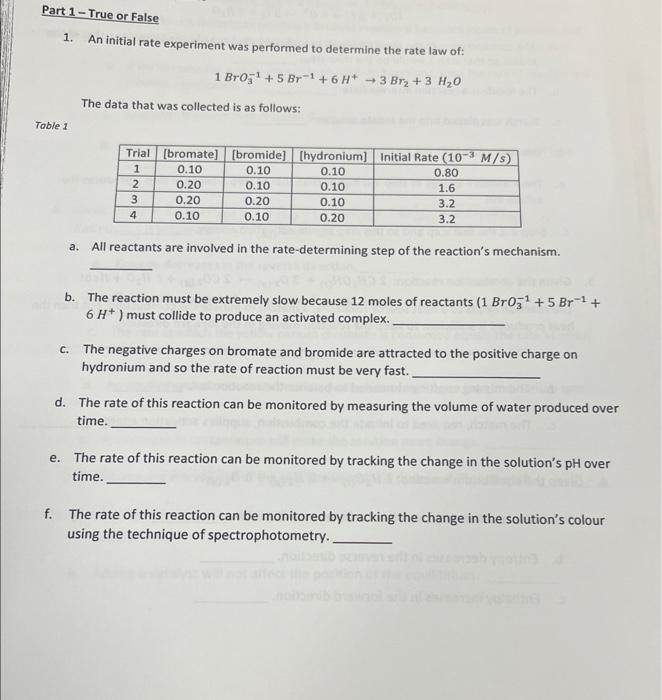

1. An initial rate experiment was performed to determine the rate law of: 1BrO31+5Br1+6H+3Br2+3H2O The data that was collected is as follows: a. All reactants are involved in the rate-determining step of the reaction's mechanism. b. The reaction must be extremely slow because 12 moles of reactants (1BrO31+5Br1+ 6H+) must collide to produce an activated complex. c. The negative charges on bromate and bromide are attracted to the positive charge on hydronium and so the rate of reaction must be very fast. d. The rate of this reaction can be monitored by measuring the volume of water produced over time. e. The rate of this reaction can be monitored by tracking the change in the solution's pH over time. f. The rate of this reaction can be monitored by tracking the change in the solution's colour using the technique of spectrophotometry. 2. In lab 18 B Part 1, we've seen the mechanism: 1O3+3HSO3+I+3SO42+3H+(rds)1O3+5I+6H++3I2+3H2O a. The concentration of the bisulfite was purposely designed to be much lower than that of the iodate so that step 1 would be the slow step. b. The lodide is a reaction intermediate. c. Starch was placed into the iodate solution. d. lodide is a catalyst. e. The activation energy of step 1 is higher than that of step 2 because step 1 produces the sulfate ion. f. Step 2 is the fast step and so this step has no activation energy. 3. Given the combustion reaction: 2CH3OH(s)+3O2(g)2CO2(g)+ ? H2O+ Heat The reaction requires a heat source (example, a match) to initiate the combustion, but then the heat produced is sufficient to keep the reaction moving in the forward direction. a. The reaction goes to completion. b. The missing coefficient needed to balance the reaction is 4 . c. The match, required to initiate the combustion, supplies activation energy. 4. Given the equilibrium reaction: 2H2O(0)2H2()+1O2(g) a. The reaction is exothermic in the reverse direction. b. Entropy decreases in the reverse direction. c. Enthalpy increases in the forward direction 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


