Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help with part 3 all parts- imp Co(H2O)62(aq)+4Cl(aq)CoCl2(aq)+6H2O(l)pinkblue Based on your observations, in which direction does the equilibrium shift (left or right?): (1 mark)
please help with part 3 all parts- imp 
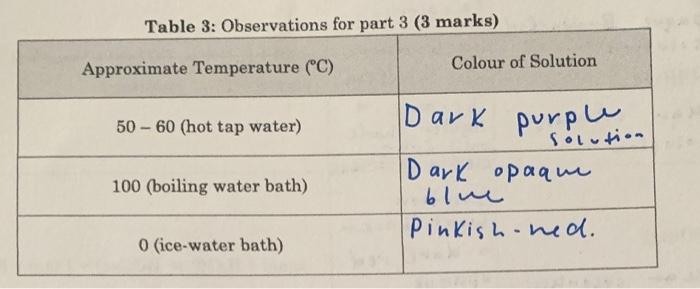
Co(H2O)62(aq)+4Cl(aq)CoCl2(aq)+6H2O(l)pinkblue Based on your observations, in which direction does the equilibrium shift (left or right?): (1 mark) when heated from 50C to approximately 100C let when cooled from 100C to approximately 0C Based on your answers to the question 6 above, decide whether the equilibrium is exothermic (H0). Explain your choice clearly. ( 2 marks) Write an expression for the equilibrium constant, K6, for the above reaction. (1 mark) When the temperature is increased, what happens to the value of K (does it increase, decrease, or stay the same)? Explain. (2 marks) Table 3: Observations for part 3 (3 marks) \begin{tabular}{|c|c|} \hline Approximate Temperature (C) & Colour of Solution \\ \hline 5060 (hot tap water) & DarK \\ \hline 100 (boiling water bath) & Dur ark opaqu \\ \hline 0 (ice-water bath) & Pinkish olol. \\ \hline \end{tabular} 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


