Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help with the Mass Spec based question below. Please explain your reasoning and please try to theorize some structures as well. Thank you 1.2.
Please help with the Mass Spec based question below.

Please explain your reasoning and please try to theorize some structures as well.
Thank you
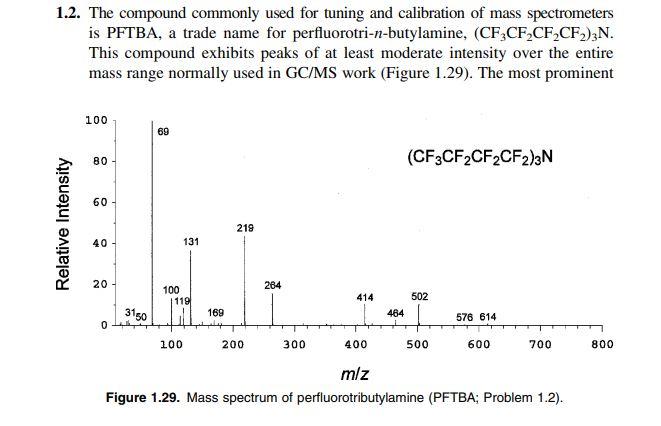
1.2. The compound commonly used for tuning and calibration of mass spectrometers is PFTBA, a trade name for perfluorotri- n-butylamine, (CF3CF2CF2CF2)3N. This compound exhibits peaks of at least moderate intensity over the entire mass range normally used in GC/MS work (Figure 1.29). The most prominent Figure 1.29. Mass spectrum of perfluorotributylamine (PFTBA; Problem 1.2). peaks in the spectrum of PFTBA occur at m/z31,69,100,114,119,131,219, 264,414,464,502,576, and 614 . Devise elemental compositions (and, if possible, hypothesize structures) for the ions that correspond to each of these peaksStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


