Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE HURRY URGENT! I WILL UPVOTE! How many grams of solid ammonium bromide should be added to 0.500L of a 0.265M ammonia solution to prepare
PLEASE HURRY URGENT! I WILL UPVOTE! 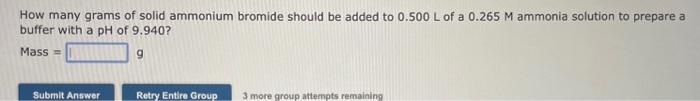
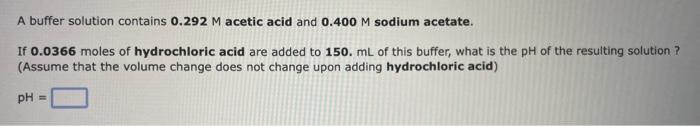
How many grams of solid ammonium bromide should be added to 0.500L of a 0.265M ammonia solution to prepare a buffer with a pH of 9.940 ? Mass = 9 A buffer solution contains 0.292M acetic acid and 0.400M sodium acetate. If 0.0366 moles of hydrochloric acid are added to 150. mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume change does not change upon adding hydrochloric acid) How many grams of solid ammonium bromide should be added to 0.500L of a 0.265M ammonia solution to prepare a buffer with a pH of 9.940 ? Mass = 9 A buffer solution contains 0.292M acetic acid and 0.400M sodium acetate. If 0.0366 moles of hydrochloric acid are added to 150. mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume change does not change upon adding hydrochloric acid) 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


