Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please I need discussion (analysis) of the data collected on the concentration versus absorbance. And also make a graph using the concentration versus absorbance. Also
please I need discussion (analysis) of the data collected on the concentration versus absorbance. And also make a graph using the concentration versus absorbance. Also discuss the challenges you face doing the experiment and the possible remedies to tackle the challenge. Also using graph to predict the concentration of substances unknown A unknown B.
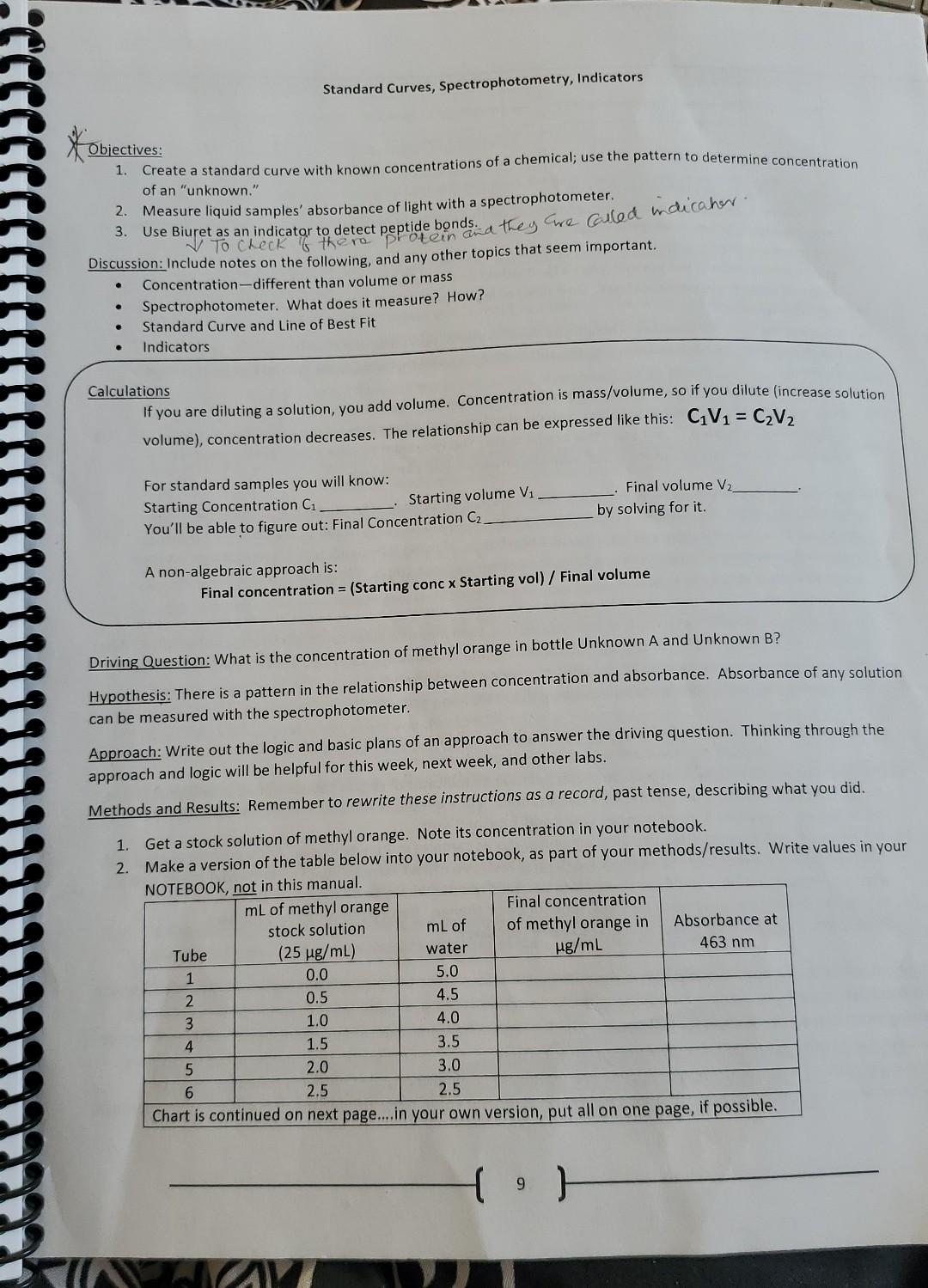

Objectives: 1. Create a standard curve with known concentrations of a chemical; use the pattern to determine concentration of an "unknown." 2. Measure liquid samples' absorbance of light with a spectrophotometer. 3. Use Biuret as an indicator to detect peptide bonds. They cwe Colled m dicahor. Discussion: Include notes on the following, and any other topics that seem important. - Concentration-different than volume or mass - Spectrophotometer. What does it measure? How? - Standard Curve and Line of Best Fit - Indicators Calculations If you are diluting a solution, you add volume. Concentration is mass/volume, so if you dilute (increase solution volume), concentration decreases. The relationship can be expressed like this: C1V1=C2V2 For standard samples you will know: Starting Concentration C1 Starting volume V1 . Final volume V2 You'll be able to figure out: Final Concentration C2 by solving for it. A non-algebraic approach is: Final concentration =( Starting conc Starting vol )/ Final volume Driving Question: What is the concentration of methyl orange in bottle Unknown A and Unknown B? Hypothesis: There is a pattern in the relationship between concentration and absorbance. Absorbance of any solution can be measured with the spectrophotometer. Approach: Write out the logic and basic plans of an approach to answer the driving question. Thinking through the approach and logic will be helpful for this week, next week, and other labs. Methods and Results: Remember to rewrite these instructions as a record, past tense, describing what you did. 1. Get a stock solution of methyl orange. Note its concentration in your notebook. 2. Make a version of the table below into your notebook, as part of your methods/results. Write values in your 5. For column 5 , use a spectrophotometer to measure absorbance. You find instructions for the spectrophotometer in this manual (page 11). It would be advisable to make a copy of the instructions for the spec and paste it into your notebook. Do it now, or leave space today and put it in before next week. You'll need it in the future! 6. Keep out the spec and cuvettes. Though you need to pause, don't disconnect or turn off the spec. 7. Graph your data, by hand and/or using the automated graphing through Logger Pro. (ask instructor what's required). 8. Add the line of best fit to your graph to show the general pattern (see directions, step 14). 9. If you are using the automated graphing, you need to keep and print a copy of the graph for your notebook. See the spec directions, step 17, for ways this can be done. You may want to save a copy before you test the unknowns, and/or versions of the graph with the unknown info plotted on. 10. For Unknown A, you can make a new place in your notebook for data. Measure A's absorbance with the spectrophotometer and note the value in your notebook. Use step 15/16 of the spec directions for the automated way to get concentration with the absorbance. If doing it by hand, plot A's absorbance on your graph and determine A's concentration from the pattern on your graph. Write down your estimated concentration of methyl orange in A. 11. Unknown B is more concentrated than any of your knowns. What can you do to get it in the range of your known concentrations? Keep track of how you do this, then measure absorbance of the adjusted solution. After plotting, use a dilution factor or the concentration/volume relationships described above to work backwards and determine the concentration of the bottled solution of unknown B. 12. You may dispose methyl orange solutions down the drain. III Safety precautions when working with Biuret solution: wear gloves, wear goggles, dispose any solutions with it in the marked waste container. 13. For your last activity today, you will use Biuret solution an indicator of peptide bonds (found in proteins). For a Biuret test, you will add 1 part Biuret to 4 parts sample. For example, if you had 4mL of a sample, you'd add 1 mL of Biuret. If you had 8mL of sample, you'd add 2mL of Biuret. 14. Put on gloves and goggles, then use albumin and water as samples in 2 control tests. For a positive control test, you expect to see a reaction. For a negative control test, you expect no reaction. Talk with your group about what you expect and how you'll set these tests up. Briefly write what you do in your notebook. 15. As you add Biuret to your control samples, look for color change. Record the colors as your data. 16. Dispose any solutions with Biuret in marked waste container. Summary: Look at your graph. Write down in the summary a reference to the page it's on (make sure you have added it to the notebook, even if you made it on the computer!). In your written summary, tell the numerical concentrations (including units) that you estimated for Unknown A and B. Explain how you determined those concentrations. Describe how Biuret can be used as an indicator of protein content. Think ahead to next week-you will be asked to design a method to determine the concentration of protein in a gummy bear, whose protein content is unknown. How can today's techniques be applied to new situations like that? Write out some basic ideas that you might try as an approach
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


