Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please I need help. thank you for this one ai have trie 120 and that is jot the answer neither If the symbol X represents
please I need help. 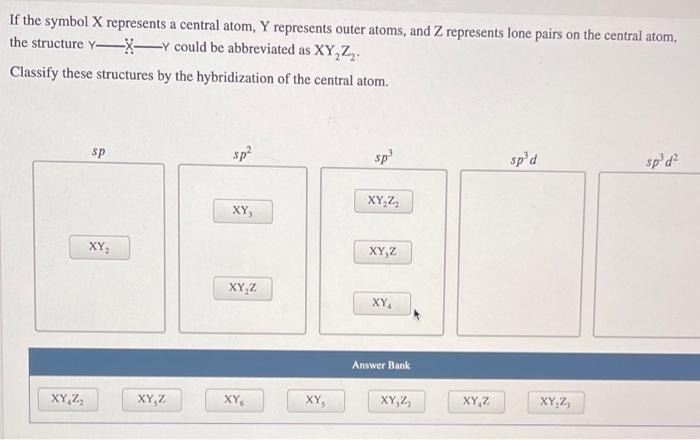
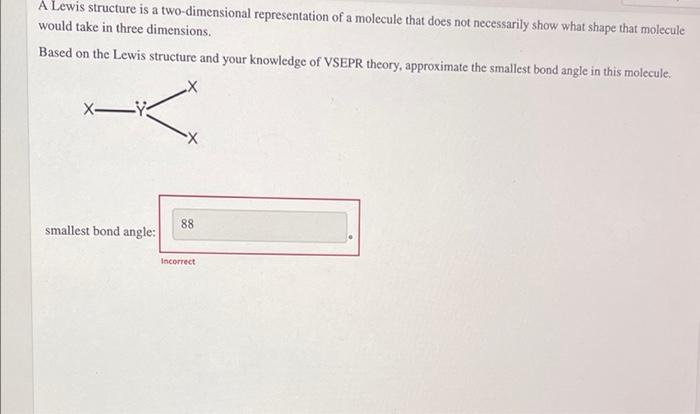
If the symbol X represents a central atom, Y represents outer atoms, and Z represents lone pairs on the central atom, the structure Xy could be abbreviated as XY, Z. Classify these structures by the hybridization of the central atom. sp sp? sp spd spod XYZ , XY, XYZ XYZ XY Answer Bank 42 XYZ XY XY XYZ XYZ XYZ A Lewis structure is a two-dimensional representation of a molecule that does not necessarily show what shape that molecule would take in three dimensions. Based on the Lewis structure and your knowledge of VSEPR theory, approximate the smallest bond angle in this molecule. X- K X 88 smallest bond angle: Incorrect thank you

for this one ai have trie 120 and that is jot the answer neither

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


