Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please make answers clear to read thank you Decide whether each chemical reaction in the table below is an oxidation-reduction (redox) reaction. If the reaction
please make answers clear to read thank you 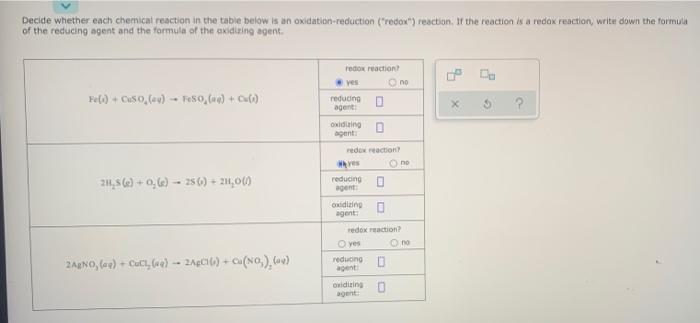
Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox") reaction. If the reaction is a redox reaction, write down the formula of the reducing agent and the formula of the oxidizing agent. Fe(1) + OS 0 (av) - 0,0) + Cul X 2 redox reaction ves no reducing agent ouding agent: red reaction ves no 21,56e) +0,6) -- 250) + 21,000 reducingo agent ondiale agent: redox reaction reducing no 2A2NO, (e) + Cut, 60) -- 2ACI(1) + Cu(NO).) ondities agent 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


