Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please match the terms pls, will be sure to leave a like if correct. Match the term with the most appropriate definition. adhesive force temperature
Please match the terms pls, will be sure to leave a like if correct. 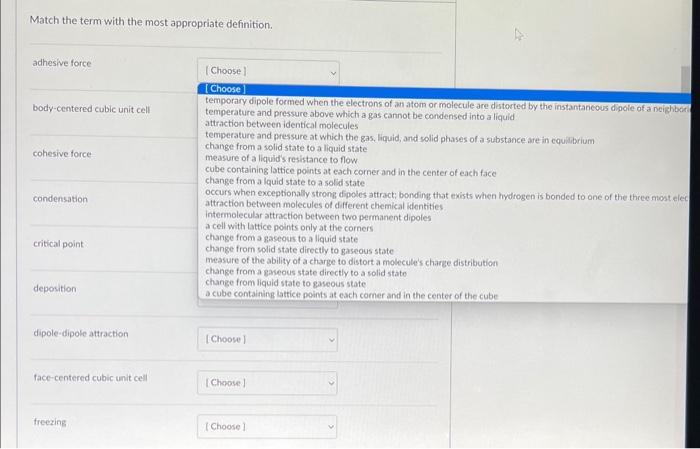
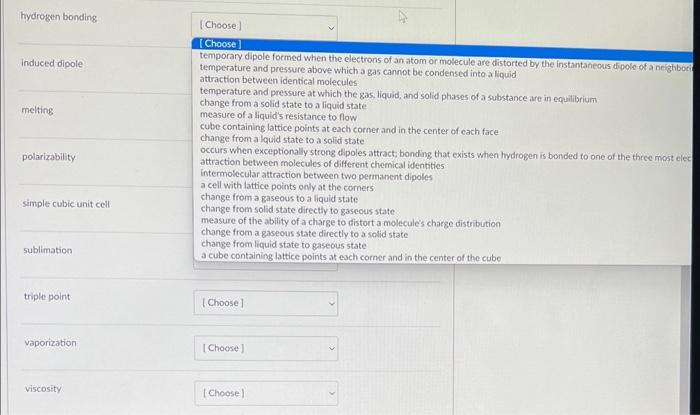
Match the term with the most appropriate definition. adhesive force temperature and pressure above which a gas cannot be condensed into a liquid attraction between identical molecules temperature and pressure at which the gas, liquid; and solid phases of a substance are in cquilbrium change from a solid state to a liquid state measure of a liquids resistance to flow. cube containing lattice points at each comerand in the center of each face change from a lquid state to a solid state occurs when exceptionally strong dipoles attract; bonding that exists when hydrogen is bonded to one of the three niost elec attraction between molecules of different chemical identities. intermolecular attraction between two permanent dipoles a cell with Lattice points only at the corners change from a gaseous to a liquid state change from solid state directly to gaseous state measure of the ability of a charge to distort a molecule's charge distribution change froma gaseous state directly to a solid state change from liquid state to gaseous state a cube containing lattice points at each comer and in the center of the cube dipole-dipole attraction face-centered cubic unit cell freezing hydrogen bonding induced dipole melting polarizability simple cubic unit cel sublimation triple point vaporization viscosity 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


