Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please note that this is a single question as indicated by the question indicator on the top right. Glycogen phosphorylase catalyzes the removal of glucose
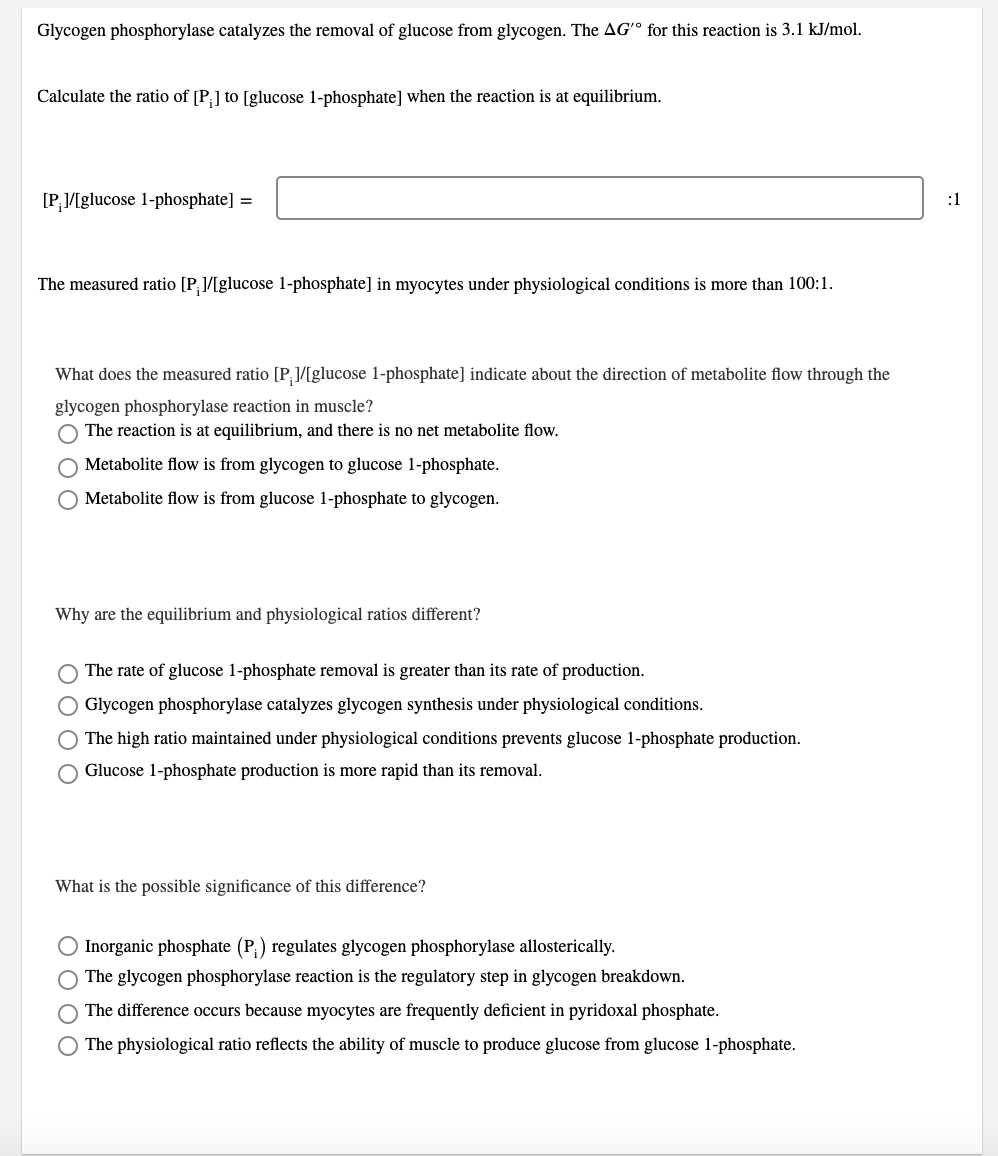
Please note that this is a single question as indicated by the question indicator on the top right.
Glycogen phosphorylase catalyzes the removal of glucose from glycogen. The AG' for this reaction is 3.1 kJ/mol. Calculate the ratio of [P] to (glucose 1-phosphate] when the reaction is at equilibrium. [P:/[glucose 1-phosphate) = :1 The measured ratio [P]/[glucose 1-phosphate) in myocytes under physiological conditions is more than 100:1. What does the measured ratio [P]/[glucose 1-phosphate) indicate about the direction of metabolite flow through the glycogen phosphorylase reaction in muscle? The reaction is at equilibrium, and there is no net metabolite flow. Metabolite flow is from glycogen to glucose 1-phosphate. Metabolite flow is from glucose 1-phosphate to glycogen. Why are the equilibrium and physiological ratios different? The rate of glucose 1-phosphate removal is greater than its rate of production. O Glycogen phosphorylase catalyzes glycogen synthesis under physiological conditions. O The high ratio maintained under physiological conditions prevents glucose 1-phosphate production. Glucose 1-phosphate production is more rapid than its removal. What is the possible significance of this difference? Inorganic phosphate (P:) regulates glycogen phosphorylase allosterically. The glycogen phosphorylase reaction is the regulatory step in glycogen breakdown. The difference occurs because myocytes are frequently deficient in pyridoxal phosphate. The physiological ratio reflects the ability of muscle to produce glucose from glucose 1-phosphateStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


