Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please provide detail answers for questions c), d) & e) (the last 3 questions). Thank you! 1) Enzyme kinetics with non-productive binding [ 80pts]. Consider

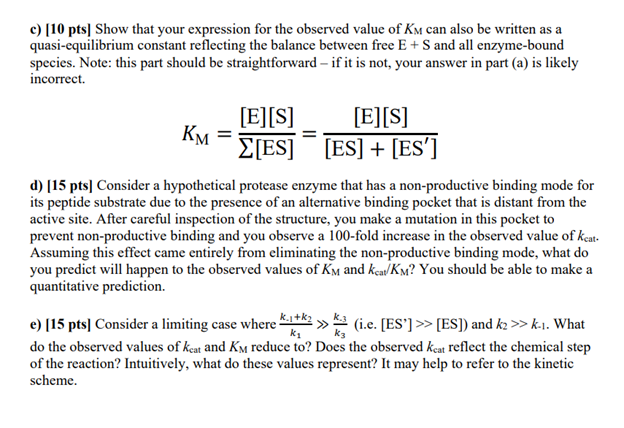
Please provide detail answers for questions c), d) & e) (the last 3 questions). Thank you!
1) Enzyme kinetics with non-productive binding [ 80pts]. Consider a kinetic scheme where the substrate can bind an enzyme in two orientations. One state (ES) can proceed to product and another state (ES') is in a non-productive conformation: E+Sk1k1ESk2E+Pk3k3ES a) [30 pts] Derive a rate equation for this reaction. Your answer should take the form: Vobs=(c2+[S])c1[E]0[S] Where c1 and c2 are constants that are functions of the individual rate constants.* c1 is the observed value of kcat and c2 is the observed value of KM. Your answer will be in the general form of the Michaelis-Menten equation and you should be able to write expressions for kcat (i.e., c1 - the observed value of kcat ) and KM (i.e., c2 - the observed value of KM ) in terms of the individual rate constants above. Hint: this and subsequent steps of the problem will be much easier if you group rate constants together in apparent equilibrium constants like the way we define KM in the simple Michaelis-Menten scheme. *Note: your answer must take the form exactly as indicated above. c1 and c2 are constants with no dependence on [S] or any other variables. You still have more algebra to do (and parts c-d will be extremely difficult) if your answer looks like this: Vobs=(cy+cz[S])+[S]cx[E]0[S] b) [10 pts] Show that your equation reduces to the familiar form of the Michaelis-Menten equation derived in class for the limiting case where k3=0. c) [10 pts] Show that your expression for the observed value of KM can also be written as a quasi-equilibrium constant reflecting the balance between free E+S and all enzyme-bound species. Note: this part should be straightforward - if it is not, your answer in part (a) is likely incorrect. KM=[ES][E][S]=[ES]+[ES][E][S] d) [15 pts] Consider a hypothetical protease enzyme that has a non-productive binding mode for its peptide substrate due to the presence of an alternative binding pocket that is distant from the active site. After careful inspection of the structure, you make a mutation in this pocket to prevent non-productive binding and you observe a 100 -fold increase in the observed value of kcat. Assuming this effect came entirely from eliminating the non-productive binding mode, what do you predict will happen to the observed values of KM and kcat/KM ? You should be able to make a quantitative prediction. e) [15 pts] Consider a limiting case where k1k1+k2k3k3 (i.e. [ES'] [ES] ) and k2k1. What do the observed values of kcat and KM reduce to? Does the observed kcat reflect the chemical step of the reaction? Intuitively, what do these values represent? It may help to refer to the kinetic schemeStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


