Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show all steps, thanks 1. The pressure gauge on a 20-ft tank of nitrogen at 75 F reads 82 psi. Estimate the mass (Ibm)

please show all steps, thanks
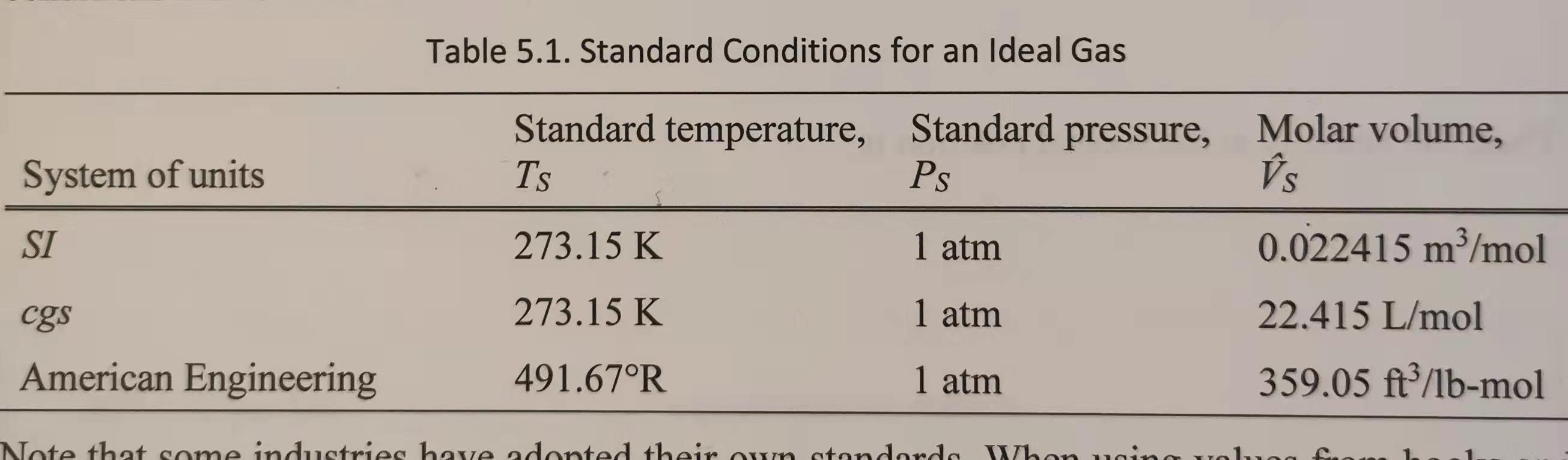
1. The pressure gauge on a 20-ft tank of nitrogen at 75 F reads 82 psi. Estimate the mass (Ibm) of nitrogen in the tank by: (a) direct solution of the ideal gas law, and (b) conversion from standard conditions. (See Table 5.1 in the Course Notes.) You must work the problem in the given units (i.e., American Engineering system of units). Do not convert to the SI or any other system of units. Table 5.1. Standard Conditions for an Ideal Gas System of units SI Standard temperature, Standard pressure, Molar volume, TS Ps is 273.15 K 1 atm 0.022415 m3/mol 273.15 K 1 atm 22.415 L/mol 491.67R 1 atm 359.05 ft/lb-mol cgs American Engineering Note that some industries have adopted their own stondendo When in yolesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


