Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please show all work and explain it. The answer is -278.9kJ. Thank you 4. One mole of gaseous A is injected at 25C into a
Please show all work and explain it.
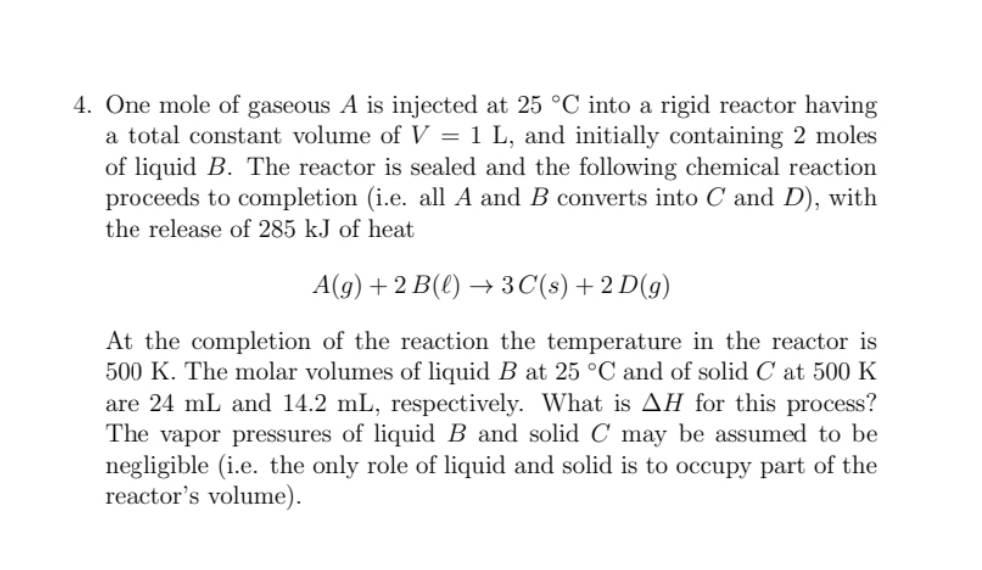
The answer is -278.9kJ. Thank you
4. One mole of gaseous A is injected at 25C into a rigid reactor having a total constant volume of V=1L, and initially containing 2 moles of liquid B. The reactor is sealed and the following chemical reaction proceeds to completion (i.e. all A and B converts into C and D ), with the release of 285kJ of heat A(g)+2B()3C(s)+2D(g) At the completion of the reaction the temperature in the reactor is 500K. The molar volumes of liquid B at 25C and of solid C at 500K are 24mL and 14.2mL, respectively. What is H for this process? The vapor pressures of liquid B and solid C may be assumed to be negligible (i.e. the only role of liquid and solid is to occupy part of the reactor's volume)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


