Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show all work Ethyl chloride vapor decomposes by the first-order reaction C2H5ClC2H4+HCl The activation energy is 249kJ/mol and the frequency factor is 1.61014s1. Find
please show all work 
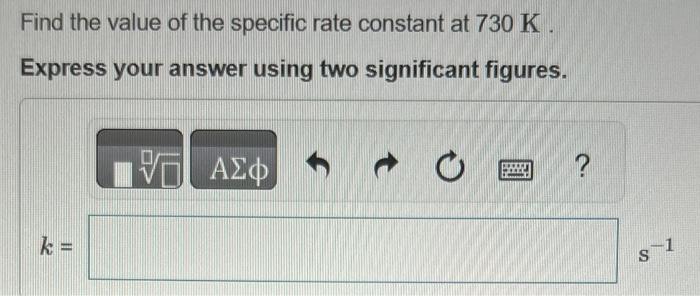
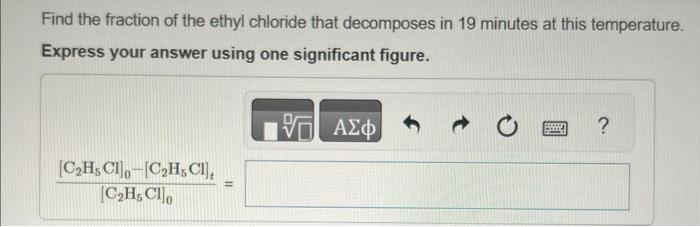
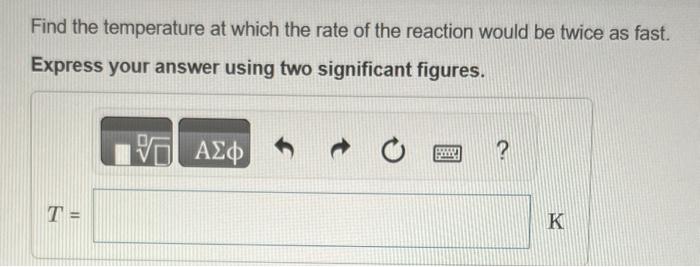
Ethyl chloride vapor decomposes by the first-order reaction C2H5ClC2H4+HCl The activation energy is 249kJ/mol and the frequency factor is 1.61014s1. Find the value of the specific rate constant at 730K. Express your answer using two significant figures. k= Find the fraction of the ethyl chloride that decomposes in 19 minutes at this temperature. Express your answer using one significant figure. Find the temperature at which the rate of the reaction would be twice as fast. Express your answer using two significant figures 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


