Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve and show all steps and calculation Bronsted-Lowry Acids and Bases (2 parts) Select the equation from the list (A-J) which illustrates the acid-base
please solve and show all steps and calculation 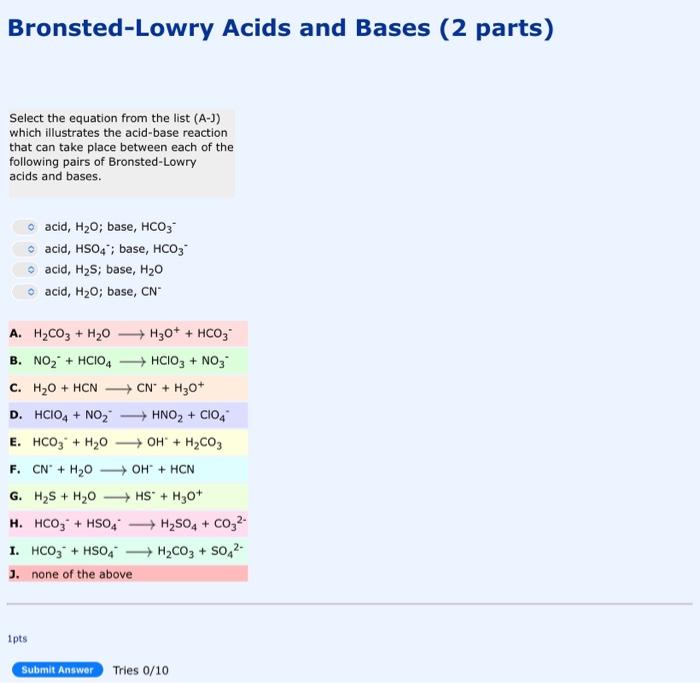
Bronsted-Lowry Acids and Bases (2 parts) Select the equation from the list (A-J) which illustrates the acid-base reaction that can take place between each of the following pairs of Bronsted-Lowry acids and bases. acid,H2O;base,HCO3acid,HSO4;base,HCO3acid,H2S;base,H2Oacid,H2O;base,CN A. H2CO3+H2OH3O++HCO3= B. NO2+HClO4HClO3+NO3 C. H2O+HCNCN+H3O+ D. HClO4+NO2HNO2+ClO4 E. HCO3+H2OOH+H2CO3 F. CN+H2OOH+HCN G. H2S+H2OHS+H3O+ H. HCO3+HSO4H2SO4+CO3 I. HCO3+HSO4H2CO3+SO42 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


