Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve by hand asap thank you 5.14 A reversible chemical reaction 2A+BC can be characterized by the equilibrium relationship K=ca2cbcc where the nomenclature ci
please solve by hand asap
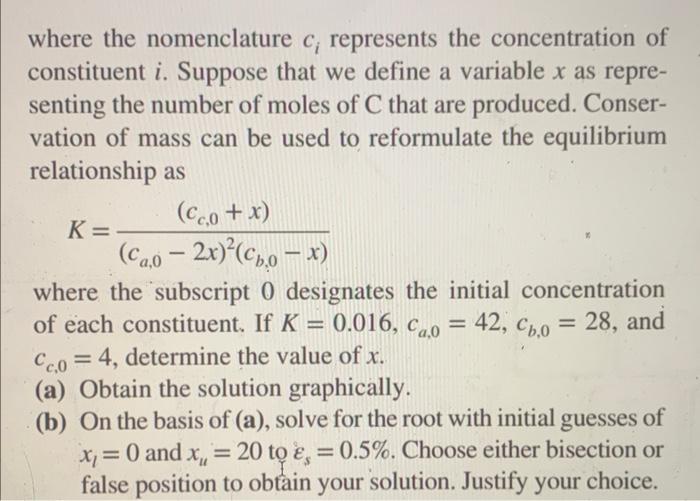
5.14 A reversible chemical reaction 2A+BC can be characterized by the equilibrium relationship K=ca2cbcc where the nomenclature ci represents the concentration of constituent i. Suppose that we define a variable x as representing the number of moles of C that are produced. Conservation of mass can be used to reformulate the equilibrium relationship as K=(ca,02x)2(cb,0x)(cc,0+x) where the subscript 0 designates the initial concentration of each constituent. If K=0.016,ca,0=42,cb,0=28, and cc,0=4, determine the value of x. (a) Obtain the solution graphically. (b) On the basis of (a), solve for the root with initial guesses of xl=0 and xu=20 to s=0.5%. Choose either bisection or false position to obtain your solution. Justify your choice thank you 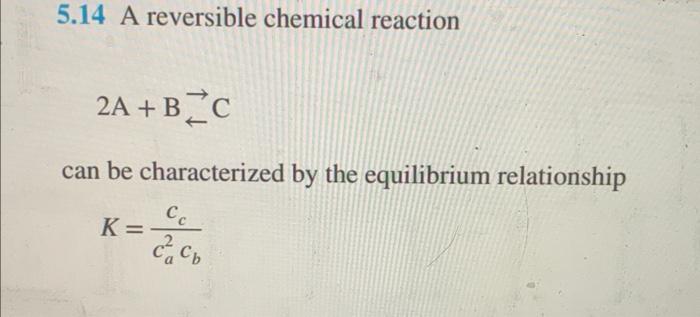
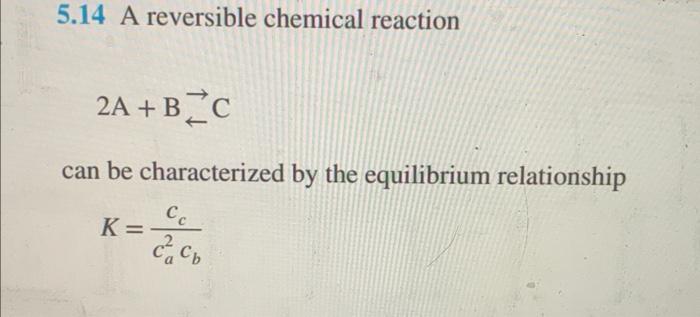

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


