Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve Gas Liquid Problem 2. Air humidification (based on WRF 27.10) Learning Objective: Use of unsteady-state time charts and heuristics for diffusion problems. A
please solve 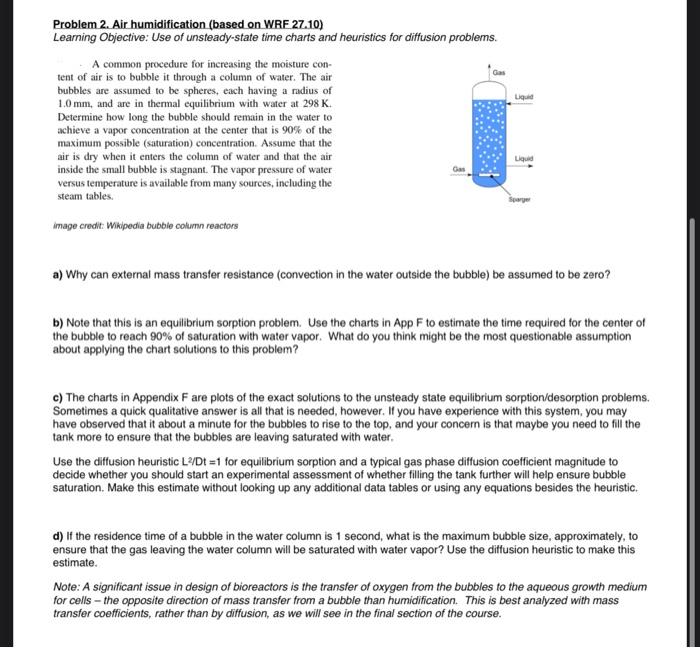
Gas Liquid Problem 2. Air humidification (based on WRF 27.10) Learning Objective: Use of unsteady-state time charts and heuristics for diffusion problems. A common procedure for increasing the moisture con- tent of air is to bubble it through a column of water. The air bubbles are assumed to be spheres, each having a radius of 1.0 mm, and are in thermal equilibrium with water at 298 K. Determine how long the bubble should remain in the water to achieve a vapor concentration at the center that is 90% of the maximum possible saturation) concentration. Assume that the air is dry when it enters the column of water and that the air inside the small bubble is stagnant. The vapor pressure of water versus temperature is available from many sources, including the steam tables Liquid Gas image credit: Wikipedia bubble column reactors a) Why can external mass transfer resistance (convection in the water outside the bubble) be assumed to be zero? b) Note that this is an equilibrium sorption problem. Use the charts in App F to estimate the time required for the center of the bubble to reach 90% of saturation with water vapor. What do you think might be the most questionable assumption about applying the chart solutions to this problem? c) The charts in Appendix F are plots of the exact solutions to the unsteady state equilibrium sorption/desorption problems. Sometimes a quick qualitative answer is all that is needed, however. If you have experience with this system, you may have observed that it about a minute for the bubbles to rise to the top, and your concern is that maybe you need to fill the tank more to ensure that the bubbles are leaving saturated with water. Use the diffusion heuristic L2/Dt =1 for equilibrium sorption and a typical gas phase diffusion coefficient magnitude to decide whether you should start an experimental assessment of whether filling the tank further will help ensure bubble saturation. Make this estimate without looking up any additional data tables or using any equations besides the heuristic. d) If the residence time of a bubble in the water column is 1 second, what is the maximum bubble size, approximately, to ensure that the gas leaving the water column will be saturated with water vapor? Use the diffusion heuristic to make this estimate. Note: A significant issue in design of bioreactors is the transfer of oxygen from the bubbles to the aqueous growth medium for cells - the opposite direction of mass transfer from a bubble than humidification. This is best analyzed with mass transfer coefficients, rather than by diffusion, as we will see in the final section of the course 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


