Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve in 40 minutes, i will surely thumb up and give 3 likes 6. Considering the following aluminothermic reaction as a way of making
please solve in 40 minutes, i will surely thumb up and give 3 likes 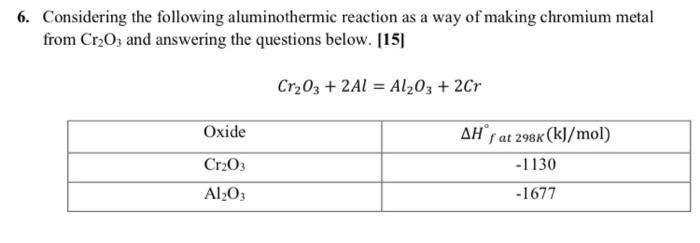
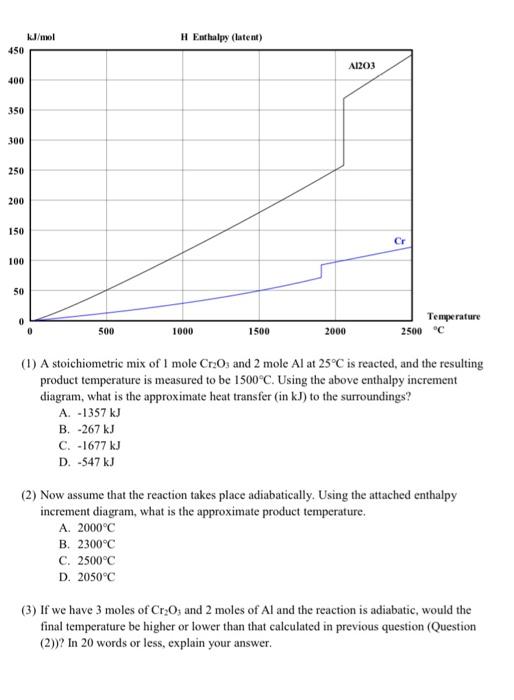
6. Considering the following aluminothermic reaction as a way of making chromium metal from Cr2O3 and answering the questions below. [15] Cr2O3 + 2A1 = Al2O3 + 2Cr Oxide Cr203 Al2O3 AH f at 298k (kJ/mol) -1130 -1677 J/mol 450 H Enthalpy (latent) A1203 400 350 300 250 200 150 Cr 100 50 0 Temperature 2500 c 500 1000 1500 2000 (1) A stoichiometric mix of 1 mole Cr2O3 and 2 mole Al at 25C is reacted, and the resulting 12 product temperature is measured to be 1500C. Using the above enthalpy increment diagram, what is the approximate heat transfer (in kJ) to the surroundings? A. -1357 kJ B. -267 kJ C. -1677 kJ D. -547 kJ (2) Now assume that the reaction takes place adiabatically. Using the attached enthalpy increment diagram, what is the approximate product temperature. A. 2000C B. 2300C C. 2500C D. 2050C (3) If we have 3 moles of Cr2O; and 2 moles of Al and the reaction is adiabatic, would the final temperature be higher or lower than that calculated in previous question (Question (2))? In 20 words or less, explain your 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


