Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve this as soon as possible Please solve all parts on word 3. A tubular wetted-wall reactor is used for the oxidation reaction O2(gl)+2B(l)P(l)
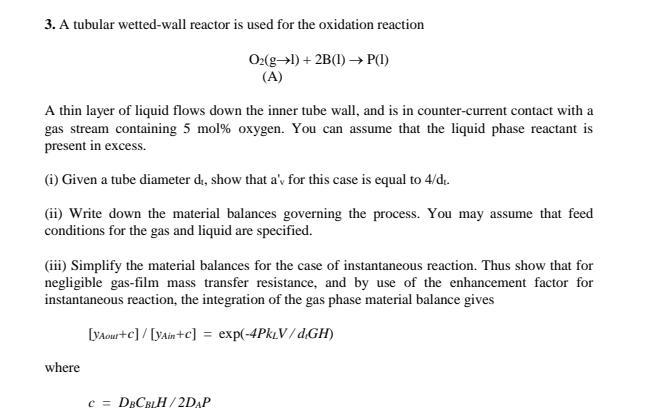 Please solve this as soon as possible
Please solve this as soon as possible
Please solve all parts on word
3. A tubular wetted-wall reactor is used for the oxidation reaction O2(gl)+2B(l)P(l) (A) A thin layer of liquid flows down the inner tube wall, and is in counter-current contact with a gas stream containing 5mol% oxygen. You can assume that the liquid phase reactant is present in excess. (i) Given a tube diameter di, show that a'v for this case is equal to 4/dt. (ii) Write down the material balances governing the process. You may assume that feed conditions for the gas and liquid are specified. (iii) Simplify the material balances for the case of instantaneous reaction. Thus show that for negligible gas-film mass transfer resistance, and by use of the enhancement factor for instantaneous reaction, the integration of the gas phase material balance gives [yAour+c]/[yAin+c]=exp(4PkLV/dlGH) where c=DBCBLH/2DAPStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


