Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please tell me which mass transfer book the following example comes from. EXAMPLE 21.4-1. Mass Transfer from Air Bubbles in Fermentation Calculate the maximum rate
Please tell me which mass transfer book the following example comes from.
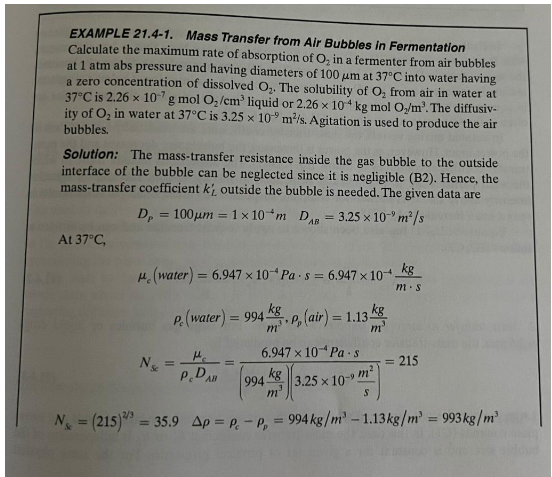
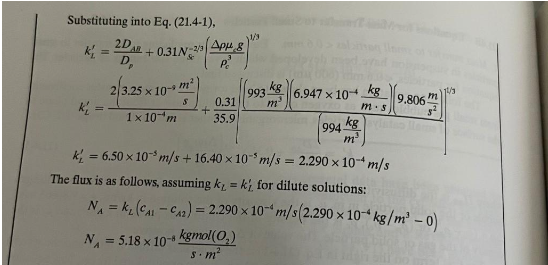 EXAMPLE 21.4-1. Mass Transfer from Air Bubbles in Fermentation Calculate the maximum rate of absorption of O2 in a fermenter from air bubbles at 1atm abs pressure and having diameters of 100m at 37C into water having a zero concentration of dissolved O2. The solubility of O2 from air in water at ity of O2 in water at 37C is 3.25109m2/s. Agitation is used to produce the air bubbles. Solution: The mass-transfer resistance inside the gas bubble to the outside interface of the bubble can be neglected since it is negligible (B2). Hence, the mass-transfer coefficient kL outside the bubble is needed. The given data are DP=100m=1104mDAB=3.25109m2/s At 37C c(water)=6.947104Pas=6.947104mskgcwater)=994m3kg,p(air)=1.13m3kgNsc=cDABc=(994m3kg)(3.25109sm2)6.947104Pas=215NS=(215)2/3=35.9=cp=994kg/m31.13kg/m3=993kg/m3 Substituting into Eq. (21.4-1), kL=Dp2DAg+0.31Nc2/3(pc3gg)1/3kL=1104m2(3.25109sm2)+35.90.31[(994m3kg)(993m3kg)(6.947104mskg)(9.806s2m)]1/3kL=6.50105m/s+16.40105m/s=2.290104m/s flux is as follows, assuming kL=kL for dilute solutions: NA=kL(cA1cA2)=2.290104m/s(2.290104kg/m30)NA=5.18108sm2kgmol(O2)
EXAMPLE 21.4-1. Mass Transfer from Air Bubbles in Fermentation Calculate the maximum rate of absorption of O2 in a fermenter from air bubbles at 1atm abs pressure and having diameters of 100m at 37C into water having a zero concentration of dissolved O2. The solubility of O2 from air in water at ity of O2 in water at 37C is 3.25109m2/s. Agitation is used to produce the air bubbles. Solution: The mass-transfer resistance inside the gas bubble to the outside interface of the bubble can be neglected since it is negligible (B2). Hence, the mass-transfer coefficient kL outside the bubble is needed. The given data are DP=100m=1104mDAB=3.25109m2/s At 37C c(water)=6.947104Pas=6.947104mskgcwater)=994m3kg,p(air)=1.13m3kgNsc=cDABc=(994m3kg)(3.25109sm2)6.947104Pas=215NS=(215)2/3=35.9=cp=994kg/m31.13kg/m3=993kg/m3 Substituting into Eq. (21.4-1), kL=Dp2DAg+0.31Nc2/3(pc3gg)1/3kL=1104m2(3.25109sm2)+35.90.31[(994m3kg)(993m3kg)(6.947104mskg)(9.806s2m)]1/3kL=6.50105m/s+16.40105m/s=2.290104m/s flux is as follows, assuming kL=kL for dilute solutions: NA=kL(cA1cA2)=2.290104m/s(2.290104kg/m30)NA=5.18108sm2kgmol(O2) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


