Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please write the whole process and describe in detail . Thanks . The Langmuir adsorption model is written as below, in which [S-M) is the
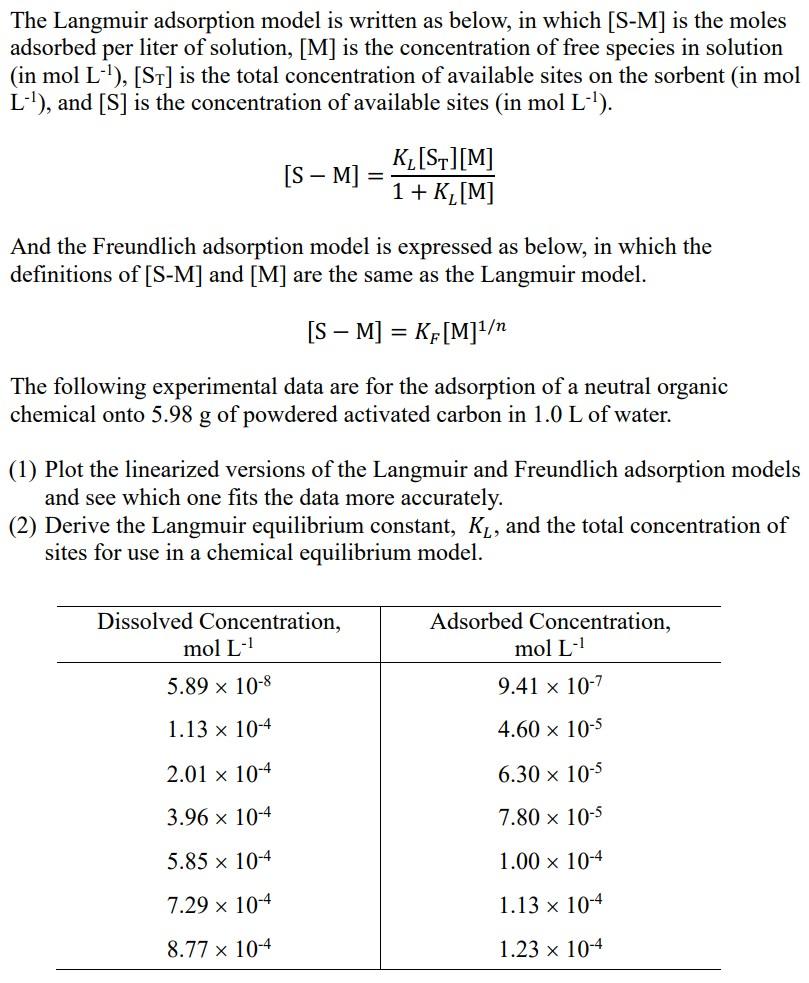
Please write the whole process and describe in detail . Thanks .
The Langmuir adsorption model is written as below, in which [S-M) is the moles adsorbed per liter of solution, [M] is the concentration of free species in solution (in mol L-'), [ST] is the total concentration of available sites on the sorbent (in mol L-1), and [S] is the concentration of available sites (in mol L-'). [S M] K_[S][M] 1 + K,[M] And the Freundlich adsorption model is expressed as below, in which the definitions of [S-M] and [M] are the same as the Langmuir model. [S M] = KF[M]1 = The following experimental data are for the adsorption of a neutral organic chemical onto 5.98 g of powdered activated carbon in 1.0 L of water. g (1) Plot the linearized versions of the Langmuir and Freundlich adsorption models and see which one fits the data more accurately. (2) Derive the Langmuir equilibrium constant, Ky, and the total concentration of sites for use in a chemical equilibrium model. Dissolved Concentration, mol L- Adsorbed Concentration, mol L- 9.41 x 10-7 5.89 x 10-8 1.13 x 10-4 4.60 x 10-5 2.01 x 10-4 6.30 x 10-5 3.96 x 10-4 7.80 x 10-5 5.85 x 10-4 1.00 x 10-4 7.29 x 10-4 1.13 x 10-4 8.77 x 10-4 1.23 x 10-4Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


