Answered step by step
Verified Expert Solution
Question
1 Approved Answer
pls answer all. I'll give you a thumbs-up if ever Directions: Assign the correct oxidation number of the individual atom in the compound or ion

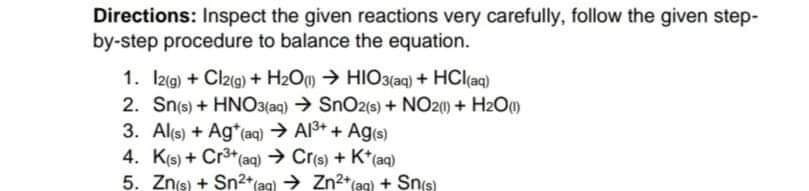

pls answer all. I'll give you a thumbs-up if ever
Directions: Assign the correct oxidation number of the individual atom in the compound or ion below. 1. S in Ss 2. Cl in CaCl2 3. I in 103 4. C in HCO3 5. Sin Fe2(SO4)3 6. Sin SO42- 7. S in Na2SO4 8. As in K3AsO4 9. Cr in Cr2O72- 10.N in NH4+ Directions: Inspect the given reactions very carefully, follow the given step- by-step procedure to balance the equation. 1. 12(g) + Cl2(g) + H2O HIO3(aq) + HCl(aq) 2. Sn(s) + HNO3(aq) SnO2(s) + NO2(1) + H20m 3. Al(s) + Ag+ (aq) A13+ + Ag(s) 4. K(s) + Cr3+ (aq) Cr(s) + K+ (aq) 5. Znis) + Sn2+(aq) Zn2+ aq) + Sn(s) Directions: Read carefully the given statements. Determine whether oxidation or reduction is described. Write your answer on the space provided. 1. An element changes oxidation number +5 to +2. 2. Oxygen attained oxidation number of O from -2. 3. Hydrogen have an initial oxidation of +1 and later had 0 after the reaction. -4. An element losses 2 electrons after a reaction. 5. Chlorine reaches - 1 charge after gaining an electron. 6. An element undergoes change of oxidation number from +3 to 0. 7. Copper changes from +2 to 0. 8. From Cl2 having O as oxidation number, CI later had -1 after combining with Na. _9. Lithium from its free state to LICI. 10. Carbon in CH4 before combustion and Carbon in CO2 after combustionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


