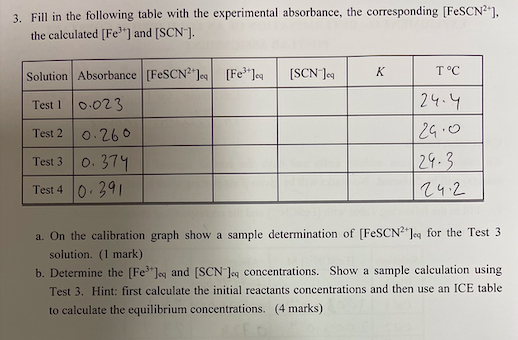
Question: pls fill the table and pls an do it asap. submission in an hour. 3. Fill in the following table with the experimental absorbance, the

![absorbance, the corresponding [FeSCN2+], the calculated [Fe3+] and [SCN]. a. On the](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6e079cb8fe_35366f6e0792cf6d.jpg)
pls fill the table and pls an do it asap. submission in an hour.
3. Fill in the following table with the experimental absorbance, the corresponding [FeSCN2+], the calculated [Fe3+] and [SCN]. a. On the calibration graph show a sample determination of [FeSCN2+]eq for the Test 3 solution. (1 mark) b. Determine the [Fe3+]q and [SCN]q concentrations. Show a sample calculation using Test 3. Hint: first calculate the initial reactants concentrations and then use an ICE table to calculate the equilibrium concentrations. (4 marks) c. Determine the value of the equilibrium constant K for all solutions. Show a sample calculation using Test 3. (1 mark) Part II: Determination of the equilibrium constant [Fe(NO3)3in1MHNO3]=2.02103M[KSCN]=1.99103M 3. Fill in the following table with the experimental absorbance, the corresponding [FeSCN2+], the calculated [Fe3+] and [SCN]. a. On the calibration graph show a sample determination of [FeSCN2+]eq for the Test 3 solution. (1 mark) b. Determine the [Fe3+]q and [SCN]q concentrations. Show a sample calculation using Test 3. Hint: first calculate the initial reactants concentrations and then use an ICE table to calculate the equilibrium concentrations. (4 marks) c. Determine the value of the equilibrium constant K for all solutions. Show a sample calculation using Test 3. (1 mark) Part II: Determination of the equilibrium constant [Fe(NO3)3in1MHNO3]=2.02103M[KSCN]=1.99103M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


