Question: including this data can someone help me with my lab? Experiment 3 Equilibrium Constants INTRODUCTION This experiment involves making measurements to perform a mathematical study





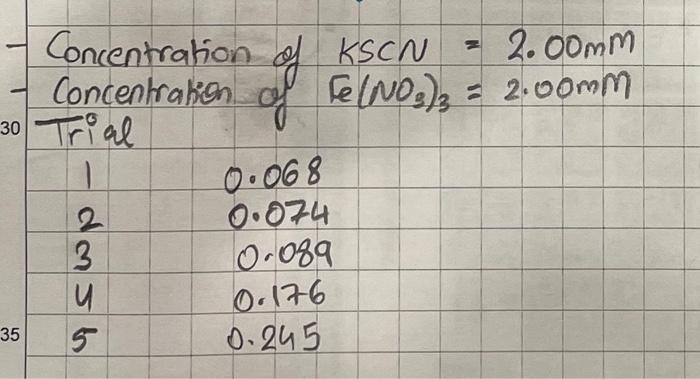
Experiment 3 Equilibrium Constants INTRODUCTION This experiment involves making measurements to perform a mathematical study of an equilibrium system. Its objectives include introducing the experimental use of pipets and the spectrophotometer, practicing the calculations used to solve an equilibrium system, and testing the constancy of the equilibrium constant. Most chemical reactions are reversible if they take place in a closed system. That is, unless the reverse reaction would require a complex collision or energy too large for the system temperature to provide, it will be possible for the accumulated products to change back into reactants. Such systems will tend to reach the equilibrium state over time. Consider a system that initially is composed of only reactant species. The reactant concentrations will provide some positive forward reaction rate. Initially, with no products present to react, the reverse reaction rate will be zero. As time passes, reactants will be consumed and products will be formed. The resulting concentration changes will reduce the forward rate and increase the reverse rate. Eventually, the forward and reverse rates will become equal and no further net concentration changes will be observed. At this point the system has reached equilibrium. One can demonstrate that, at a given temperature, there will be a constant, K, equal to the mathematical product of all chemical product concentrations raised to powers equal to their net reaction coefficients divided by the product of the reactant concentrations raised to powers matching their reaction coefficients. We will study the net reaction Fe3+(aq)+SCN(aq)FeSCN2+(aq)(colorless)(brown) which occurs in one-stepm. The forward reaction forms a weak bond between the reactant ions. Thus the reverse process breaking the bond should balance it as soon as substantial product has been formed. Since all species involved have coefficients equal to 1, the equilibrium constant expression for this reaction is Ke=[Fe3+][SCN][FeSCN2+] Lab 3 - Equilibrium Constants become established. The initial concentrations in the mixtures can be found from the through calculations of the dilutions of each component. In order to evaluate the equilibrium constant for a given trial, it is necessary to measure the concentration of at least one of the reaction species after the system reaches equilibrium, in order to determine "how far" the reaction has run from its initial state. To make this measurement, we will take advantage of the fact that the product species is colored (meaning it absorbs some light wavelengths and transmits all others). Under certain conditions, the concentration of a solution species that absorbs light can be measured by passing a beam of light through the solution and determining the fraction of the light that can be detected passing through without being absorbed. This fraction is usually expressed as a percent and referred to as the percent transmittance, %T. The absorbance, A, of light by the solution species is defined as A=log(%T100%) where the log is in base ten. An instrument designed to measure absorbance of light by solutions is called a spectrophotometer. Its main features are a lamp that acts as a light source, a monochrometer that allows the selection of a narrow wavelength band to reach the sample, and a detector (photometer) that measures the intensity of light that makes it through the sample. Adjusting dials are used to set the percent transmittance to 100% for a blank sample solution that contains zero concentration of the species to be measured, and to 0% when the light path is blocked so that no intensity reaches the detector. A simpler version of a spectrophotometer is a colorimeter - rather than measuring at only one wavelength (which can be chosen across a wide range) and measuring the percent transmittance, it measures at fixed wavelengths and reads out only absorbance of light. For this lab, you will be using the digital colorimeter, rather than the large analog spectrophotometer. At low concentrations, absorbance is proportional to the distance the light travels through the sample, b, the concentration of the absorbing species, C, and a constant for the species indicating how efficiently it absorbs light at the set wavelength, , the absorptivity: A=bC In this equation, the units of and C are made to be consistent, usually molar absorptivity and molarity respectively. In order to be able to use absorbance measurements to determine concentrations, the product ab must be held constant and its value must be found. Since ordinary lab equipment such as test tubes are not made with uniform dimensions, samples are tested in special containers called cuvets. They are manufactured to consistent dimensions and are made from a type of glass or plastic that is transparent over a wider wavelength range than Pyrex-type glass. The use of cuvets gives a constant path length (most often 1cm to make calculations easy). The value of is then found by preparing solutions of known concentration and measuring their absorbances, in order to determine the linear relationship between the absorbance and the concentration. For this lab, you will not need to determine this relationship, it will instead be given to you. When FeSCN2+ is measured in solutions using a colorimeter through a 1cm path at 470nm, this yields the relation A=(4622L/mol)C Using this relation, we can find the equilibrium concentration of FeSCN2+ resulting from each trial by collecting absorbance data. This will provide sufficient information to solve the equilibrium system and determine values for the equilibrium constant. MATERIALS AND SAFETY The solutions used in the study present no special hazards. Waste can be disposed of in the sinks. Wear goggles at all times. The equipment used requires some special care. Pipets are fragile, particularly at their fine tips. Use them as demonstrated by the instructor and when idle, set them down where they will not roll off the bench top. When using a suction bulb, do not insert the pipet top into it beyond the minimum necessary to make an air-tight seal. Depending on the bulb, it may not "stick" onto the pipet, you must instead hold it sealed on the end yourself. Always support the pipet, and always read the markings on the transfer-pipet when dispensing solutions - some allow you to draw up a specific volume, others require you to dispense a specific volume. The colorimeters used will be at your lab bench and you will have two cuvets available. Be sure to touch only the rough sides of the cuvet, not the smooth sides, and always insert the cap before insert the cuvet into the instrument. Set up five clean, 6-inch (medium-sized) test tubes in your rack for the five trials below and keep them organized by position or by labeling. Use two clean, dry, 50mL beakers to obtain 3035mL of stock Fe(NO3)3 solution and 2025mL of stock KSCN solution from the reservoirs. Record the concentrations of these solutions. Obtain two graduated, 5mL pipets and a suction bulb from the equipment cart. Designate the use of 1 pipet for 1 solution only by labeling the pipets above the markings; this cuts down on rinsing, cleaning, and contamination. If you have to use the same pipet for multiple solutions, you will have to condition it in order to maintain correct dilutions. (To condition: rinse with a small portion of the solution to be used - discard the rinse.) Using the appropriate pipet for each stock solution, deliver the volumes of solutions specified in the table below into one test tube for each trial. After the Fe(NO3)3 and KSCNN have been added, rinse the pipets and use one to add the specified volumes of distilled water to make the total volume in each test tube 10mL. Mix each solution by swirling the contents of each test tube briefly. Allow each solution to sit and react for ten minutes or more. To prepare the colorimeter, you must first "blank" it. This only needs to be done once at the beginning of the experiment. Fill one of your cuvets with deionized water. Ensure the wavelength is set correctly (given in introduction), then insert the blank solution cuvet in the colorimeter and press and hold the "calibrate" button on the colorimeter until the red light underneath stops flashing. Then test your solutions using the other cuvet. Start with trial 1. Use your solution to rinse the cuvet with a very small portion, then fill the cuvet to at least 3/4 full with your solution. Dry the outside of the cuvet if necessary using a lab-tissue wipe, NOT a paper towel. Place the cuvet in the sample chamber and close the lid. Read and record the value of absorbance. Take out the cuvet and discard the measured solution in the waste beaker. Repeat the rinsing and testing using the same cuvet for the remaining trials. Work in numerical order in order to minimize contamination. When finished, check your absorbance measurements to ensure that they make sense. If they don't, consider retesting your solutions. Once you are confident in your measurements, clean up and put away the equipment. Be sure to return the pipets and bulb to the cart. Data Be sure copy the table with the solution creating instructions into your notebook BEFORE LAB so you have the correct instructions. Record the stock concentrations of solutions. Record the absorbance of each trial neatly in a table. Completing the Lab Report Data Create both tables (or combine into one table) from your lab notebook in your report. Calculations You will need to show your work for determining the following pieces of information: Initial concentrations of iron(III) ion and thiocyanate ion in each trial ( 6 calculations - I recommend typing out one and then copy pasting) Equilibrium concentrations of the thiocyanato iron(III) complex (from avg Abs) ( 5 calculations) ICE tables for EACH trial to determine the K for EACH trial (see canvas for a template table). Average value of K across all trials Average deviation of K values (For each trial, find the absolute value of the difference between the trial K and the average K. These are the deviations from the average (the mean). Take the average of the five differences and you have the average deviation. While not as commonly used as standard deviation, the average deviation is sometimes written after a reported value using the symbol to indicate precision. This gives more information than merely rounding off to the last significant figure.) Post Lab How precise were your calculated values of the equilibrium constant for this reaction? Comment on possible sources of error. What does the value of K tell you about this reaction? Be sure to include your average K the average deviation somewhere obvious as your "summary of results table". (Since it is the only thing we are calculating, your summary of results table should include only this piece of information.) - Concentration of KSCN=2.00mm - Concentration of Fe(NO3)3=2.00mm 30 Trial \begin{tabular}{|cc} 1 & 0.068 \\ \hline 2 & 0.074 \\ 3 & 0.089 \\ 4 & 0.176 \\ 5 & 0.245 \end{tabular} Experiment 3 Equilibrium Constants INTRODUCTION This experiment involves making measurements to perform a mathematical study of an equilibrium system. Its objectives include introducing the experimental use of pipets and the spectrophotometer, practicing the calculations used to solve an equilibrium system, and testing the constancy of the equilibrium constant. Most chemical reactions are reversible if they take place in a closed system. That is, unless the reverse reaction would require a complex collision or energy too large for the system temperature to provide, it will be possible for the accumulated products to change back into reactants. Such systems will tend to reach the equilibrium state over time. Consider a system that initially is composed of only reactant species. The reactant concentrations will provide some positive forward reaction rate. Initially, with no products present to react, the reverse reaction rate will be zero. As time passes, reactants will be consumed and products will be formed. The resulting concentration changes will reduce the forward rate and increase the reverse rate. Eventually, the forward and reverse rates will become equal and no further net concentration changes will be observed. At this point the system has reached equilibrium. One can demonstrate that, at a given temperature, there will be a constant, K, equal to the mathematical product of all chemical product concentrations raised to powers equal to their net reaction coefficients divided by the product of the reactant concentrations raised to powers matching their reaction coefficients. We will study the net reaction Fe3+(aq)+SCN(aq)FeSCN2+(aq)(colorless)(brown) which occurs in one-stepm. The forward reaction forms a weak bond between the reactant ions. Thus the reverse process breaking the bond should balance it as soon as substantial product has been formed. Since all species involved have coefficients equal to 1, the equilibrium constant expression for this reaction is Ke=[Fe3+][SCN][FeSCN2+] Lab 3 - Equilibrium Constants become established. The initial concentrations in the mixtures can be found from the through calculations of the dilutions of each component. In order to evaluate the equilibrium constant for a given trial, it is necessary to measure the concentration of at least one of the reaction species after the system reaches equilibrium, in order to determine "how far" the reaction has run from its initial state. To make this measurement, we will take advantage of the fact that the product species is colored (meaning it absorbs some light wavelengths and transmits all others). Under certain conditions, the concentration of a solution species that absorbs light can be measured by passing a beam of light through the solution and determining the fraction of the light that can be detected passing through without being absorbed. This fraction is usually expressed as a percent and referred to as the percent transmittance, %T. The absorbance, A, of light by the solution species is defined as A=log(%T100%) where the log is in base ten. An instrument designed to measure absorbance of light by solutions is called a spectrophotometer. Its main features are a lamp that acts as a light source, a monochrometer that allows the selection of a narrow wavelength band to reach the sample, and a detector (photometer) that measures the intensity of light that makes it through the sample. Adjusting dials are used to set the percent transmittance to 100% for a blank sample solution that contains zero concentration of the species to be measured, and to 0% when the light path is blocked so that no intensity reaches the detector. A simpler version of a spectrophotometer is a colorimeter - rather than measuring at only one wavelength (which can be chosen across a wide range) and measuring the percent transmittance, it measures at fixed wavelengths and reads out only absorbance of light. For this lab, you will be using the digital colorimeter, rather than the large analog spectrophotometer. At low concentrations, absorbance is proportional to the distance the light travels through the sample, b, the concentration of the absorbing species, C, and a constant for the species indicating how efficiently it absorbs light at the set wavelength, , the absorptivity: A=bC In this equation, the units of and C are made to be consistent, usually molar absorptivity and molarity respectively. In order to be able to use absorbance measurements to determine concentrations, the product ab must be held constant and its value must be found. Since ordinary lab equipment such as test tubes are not made with uniform dimensions, samples are tested in special containers called cuvets. They are manufactured to consistent dimensions and are made from a type of glass or plastic that is transparent over a wider wavelength range than Pyrex-type glass. The use of cuvets gives a constant path length (most often 1cm to make calculations easy). The value of is then found by preparing solutions of known concentration and measuring their absorbances, in order to determine the linear relationship between the absorbance and the concentration. For this lab, you will not need to determine this relationship, it will instead be given to you. When FeSCN2+ is measured in solutions using a colorimeter through a 1cm path at 470nm, this yields the relation A=(4622L/mol)C Using this relation, we can find the equilibrium concentration of FeSCN2+ resulting from each trial by collecting absorbance data. This will provide sufficient information to solve the equilibrium system and determine values for the equilibrium constant. MATERIALS AND SAFETY The solutions used in the study present no special hazards. Waste can be disposed of in the sinks. Wear goggles at all times. The equipment used requires some special care. Pipets are fragile, particularly at their fine tips. Use them as demonstrated by the instructor and when idle, set them down where they will not roll off the bench top. When using a suction bulb, do not insert the pipet top into it beyond the minimum necessary to make an air-tight seal. Depending on the bulb, it may not "stick" onto the pipet, you must instead hold it sealed on the end yourself. Always support the pipet, and always read the markings on the transfer-pipet when dispensing solutions - some allow you to draw up a specific volume, others require you to dispense a specific volume. The colorimeters used will be at your lab bench and you will have two cuvets available. Be sure to touch only the rough sides of the cuvet, not the smooth sides, and always insert the cap before insert the cuvet into the instrument. Set up five clean, 6-inch (medium-sized) test tubes in your rack for the five trials below and keep them organized by position or by labeling. Use two clean, dry, 50mL beakers to obtain 3035mL of stock Fe(NO3)3 solution and 2025mL of stock KSCN solution from the reservoirs. Record the concentrations of these solutions. Obtain two graduated, 5mL pipets and a suction bulb from the equipment cart. Designate the use of 1 pipet for 1 solution only by labeling the pipets above the markings; this cuts down on rinsing, cleaning, and contamination. If you have to use the same pipet for multiple solutions, you will have to condition it in order to maintain correct dilutions. (To condition: rinse with a small portion of the solution to be used - discard the rinse.) Using the appropriate pipet for each stock solution, deliver the volumes of solutions specified in the table below into one test tube for each trial. After the Fe(NO3)3 and KSCNN have been added, rinse the pipets and use one to add the specified volumes of distilled water to make the total volume in each test tube 10mL. Mix each solution by swirling the contents of each test tube briefly. Allow each solution to sit and react for ten minutes or more. To prepare the colorimeter, you must first "blank" it. This only needs to be done once at the beginning of the experiment. Fill one of your cuvets with deionized water. Ensure the wavelength is set correctly (given in introduction), then insert the blank solution cuvet in the colorimeter and press and hold the "calibrate" button on the colorimeter until the red light underneath stops flashing. Then test your solutions using the other cuvet. Start with trial 1. Use your solution to rinse the cuvet with a very small portion, then fill the cuvet to at least 3/4 full with your solution. Dry the outside of the cuvet if necessary using a lab-tissue wipe, NOT a paper towel. Place the cuvet in the sample chamber and close the lid. Read and record the value of absorbance. Take out the cuvet and discard the measured solution in the waste beaker. Repeat the rinsing and testing using the same cuvet for the remaining trials. Work in numerical order in order to minimize contamination. When finished, check your absorbance measurements to ensure that they make sense. If they don't, consider retesting your solutions. Once you are confident in your measurements, clean up and put away the equipment. Be sure to return the pipets and bulb to the cart. Data Be sure copy the table with the solution creating instructions into your notebook BEFORE LAB so you have the correct instructions. Record the stock concentrations of solutions. Record the absorbance of each trial neatly in a table. Completing the Lab Report Data Create both tables (or combine into one table) from your lab notebook in your report. Calculations You will need to show your work for determining the following pieces of information: Initial concentrations of iron(III) ion and thiocyanate ion in each trial ( 6 calculations - I recommend typing out one and then copy pasting) Equilibrium concentrations of the thiocyanato iron(III) complex (from avg Abs) ( 5 calculations) ICE tables for EACH trial to determine the K for EACH trial (see canvas for a template table). Average value of K across all trials Average deviation of K values (For each trial, find the absolute value of the difference between the trial K and the average K. These are the deviations from the average (the mean). Take the average of the five differences and you have the average deviation. While not as commonly used as standard deviation, the average deviation is sometimes written after a reported value using the symbol to indicate precision. This gives more information than merely rounding off to the last significant figure.) Post Lab How precise were your calculated values of the equilibrium constant for this reaction? Comment on possible sources of error. What does the value of K tell you about this reaction? Be sure to include your average K the average deviation somewhere obvious as your "summary of results table". (Since it is the only thing we are calculating, your summary of results table should include only this piece of information.) - Concentration of KSCN=2.00mm - Concentration of Fe(NO3)3=2.00mm 30 Trial \begin{tabular}{|cc} 1 & 0.068 \\ \hline 2 & 0.074 \\ 3 & 0.089 \\ 4 & 0.176 \\ 5 & 0.245 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


