Answered step by step
Verified Expert Solution
Question
1 Approved Answer
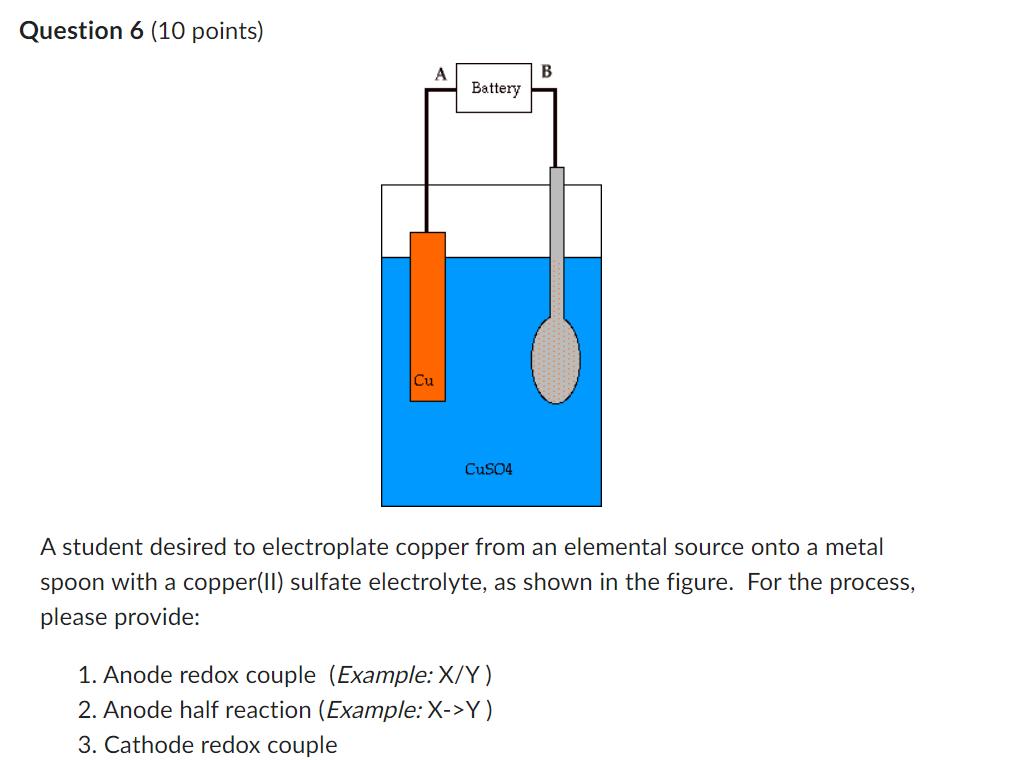
pls helppp Question 6 (10 points) A student desired to electroplate copper from an elemental source onto a metal spoon with a copper(II) sulfate electrolyte,
pls helppp
Question 6 (10 points) A student desired to electroplate copper from an elemental source onto a metal spoon with a copper(II) sulfate electrolyte, as shown in the figure. For the process, please provide: 1. Anode redox couple (Example: X/Y ) 2. Anode half reaction (Example: X>Y ) 3. Cathode redox couple 3. Cathode redox couple 4. Cathode half reaction Please use the examples as templates for your answers. Do not worry about phase, but do include charge, as appropriate. Presume that the metal of the spoon is not electrochemically active under these conditions. Blank \# 1 Blank \# 2 Blank \# 3 Blank \# 4Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


