Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Pls make a sketch first B. Two containers contain a mixture of helium and nitrogen gas, both containers are at a pressure of 2 atm
Pls make a sketch first 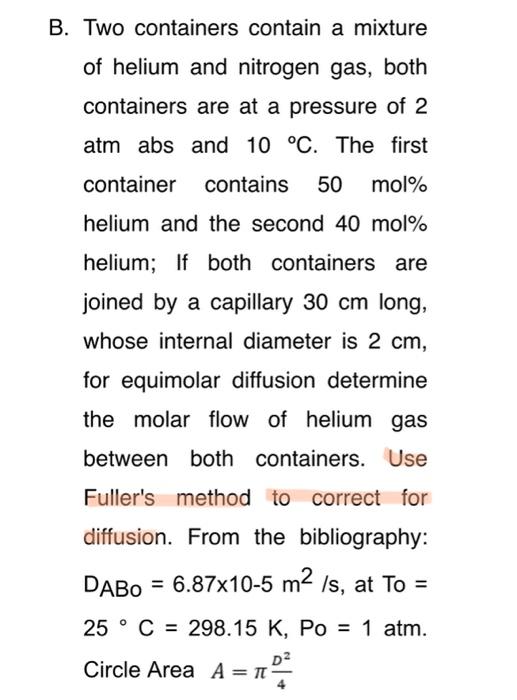
B. Two containers contain a mixture of helium and nitrogen gas, both containers are at a pressure of 2 atm abs and 10 C. The first container contains 50 mol% helium and the second 40 mol% helium; If both containers are joined by a capillary 30 cm long, whose internal diameter is 2 cm, for equimolar diffusion determine the molar flow of helium gas between both containers. Use Fuller's method to correct for diffusion. From the bibliography: DABO = 6.87x10-5 m2 is, at To = 25 C = 298.15 K, PO = 1 atm. D2 Circle Area A=1 T 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


