Answered step by step
Verified Expert Solution
Question
1 Approved Answer
plz all For the reaction H2S(g)+2H2O(I)3H2(g)+SO2(g) H=295kJ and S=295J/K G for this reaction would be negative at temperatures (above, below) K Enter above or below
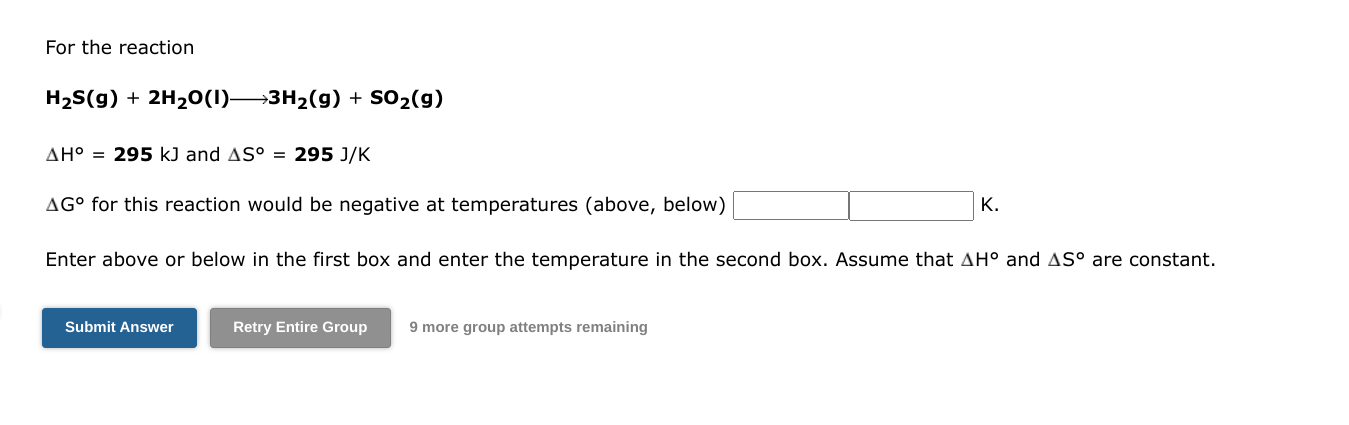

plz all
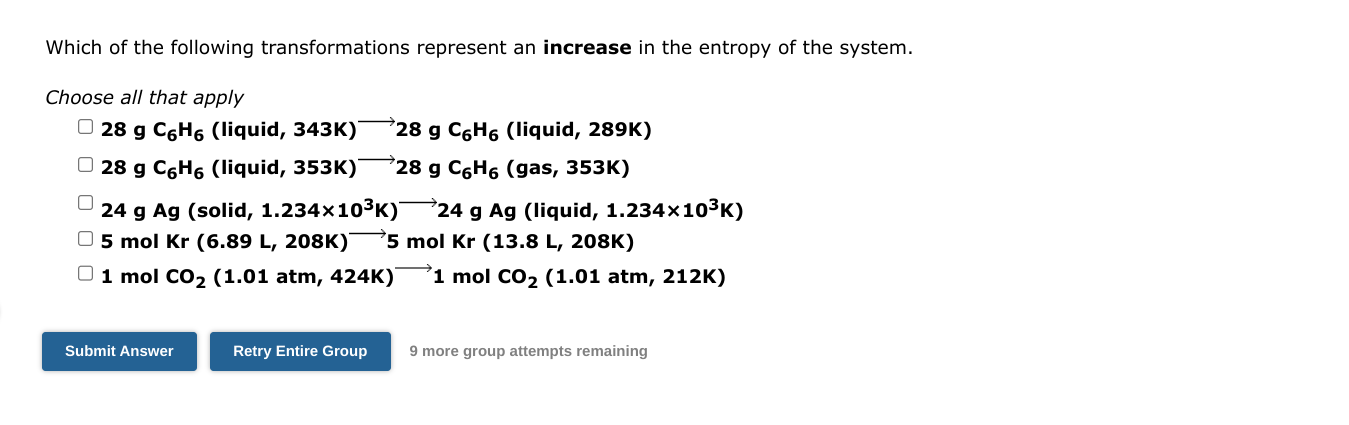
For the reaction H2S(g)+2H2O(I)3H2(g)+SO2(g) H=295kJ and S=295J/K G for this reaction would be negative at temperatures (above, below) K Enter above or below in the first box and enter the temperature in the second box. Assume that H and S are constant. 9 more group attempts remaining Which of the following transformations represent an increase in the entropy of the system. Choose all that apply 28gC6H6(liquid,343K)28gC6H6(liquid,289K)28gC6H6(liquid,353K)28gC6H6(gas,353K)24gAg(solid,1.234103K)24gAg(liquid,1.234103K)5molKr(6.89L,208K)5molKr(13.8L,208K)1molCO2(1.01atm,424K)1molCO2(1.01atm,212K) 9 more group attempts remaining Consider the reaction: H2(g)+C2H4(g)C2H6(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.71 moles of H2(g) react at standard conditionsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


