Answered step by step
Verified Expert Solution
Question
1 Approved Answer
plz with explination The Lennard-Jones potential EN=EA+ER=4(r)6+4(r)12 gives the energy of nobel gases. (a) Derive an analytical expression for the equilibrium distance r0 as a
plz with explination 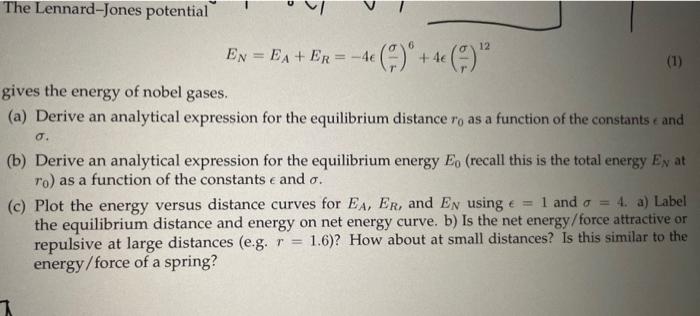
The Lennard-Jones potential EN=EA+ER=4(r)6+4(r)12 gives the energy of nobel gases. (a) Derive an analytical expression for the equilibrium distance r0 as a function of the constants c and . (b) Derive an analytical expression for the equilibrium energy E0 (recall this is the total energy EN at r0 ) as a function of the constants and . (c) Plot the energy versus distance curves for EA,ER, and EN using =1 and =4. a) Label the equilibrium distance and energy on net energy curve. b) Is the net energy/force attractive or repulsive at large distances (e.g. r=1.6 )? How about at small distances? Is this similar to the energy/force of a spring? The Lennard-Jones potential EN=EA+ER=4(r)6+4(r)12 gives the energy of nobel gases. (a) Derive an analytical expression for the equilibrium distance r0 as a function of the constants c and . (b) Derive an analytical expression for the equilibrium energy E0 (recall this is the total energy EN at r0 ) as a function of the constants and . (c) Plot the energy versus distance curves for EA,ER, and EN using =1 and =4. a) Label the equilibrium distance and energy on net energy curve. b) Is the net energy/force attractive or repulsive at large distances (e.g. r=1.6 )? How about at small distances? Is this similar to the energy/force of a spring 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


