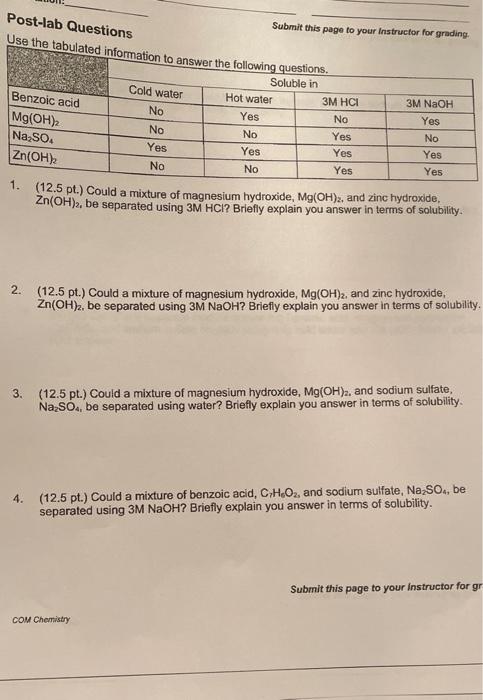
Post-lab Questions Use the tabulated information to answer the following questions. Submit this page to your instructor for grading Cold water 3M NaOH No Benzoic acid Mg(OH)2 Na2SO4 Zn(OH), No Yes Soluble in Hot water 3M HCI Yes No No Yes Yes Yes No Yes Yes No Yes Yes No 1. (12.5 pt.) Could a mixture of magnesium hydroxide, Mg(OH)2, and zinc hydroxide, Zn(OH), be separated using 3M HCI? Briefly explain you answer in terms of solubility. 2. (12.5 pt.) Could a mixture of magnesium hydroxide, Mg(OH)2, and zinc hydroxide, Zn(OH)2, be separated using 3M NaOH? Briefly explain you answer in terms of solubility. 3. (12.5 pt.) Could a mixture of magnesium hydroxide, Mg(OH), and sodium sulfate, Na SO, be separated using water? Briefly explain you answer in terms of solubility. 4. (12.5 pt.) Could a mixture of benzoic acid, CH.02, and sodium sulfate, Na, SO., be separated using 3M NaOH? Briefly explain you answer in terms of solubility. Submit this page to your instructor for gr COM Chemistry page to your Instructor for grading. Post-lab Questions 5. (15 pt.) What solvent can be used to separate benzoic acid from sodium sulfate, Na2SO.? Briefly explain your answer in terms of solubility. 6. (15 pt.) Explain how you would recover the benzoic acid and the sodium sulfate Na SO.. 7. (20 pt.) Could you separate mixture containing benzoic acid, magnesium hydroxide, Mg(OH)2, and sodium sulfate Na2SO. using only cold water and 3M HCI? Briefly explain your answer. operating the Components of Tomory Mature Group Members (print): Group Members (print): Lab Station: Calculations and Conclusions Preparing the Mixture for Separation Submit this page to your instructor for grading. Mass of beaker 1 Mass of beaker 1 and ternary mixture Mass of ternary mixture Recovering the Sodium chloride Mass of beaker 2 Mass of beaker 2 and sodium chloride (NaCl) Mass of sodium chloride (NaCl) Recovering the Sand 9 Mass of evaporating dish g Mass of evaporating dish and sand (SiO2) g Mass of sand (SIO2) . Recovering the Calcium carbonate g Mass of watch glass with filter paper Mass of watch glass, filter paper, and calcium carbonate (Caco) . Mass of calcium carbonate (CaCO.) 9 Work Space Calculations and Conclusions Recovering the Calcium carbonate Submit this page to your instructor for grading Total mass of recovered NaCl, SiO2, and Caco, Percent NaCl in mixture 9 % Percent SiO2 in mixture 29 % Percent Caco, in mixture % Percent error for recovery percentage % Work Space Observations and Conclusions