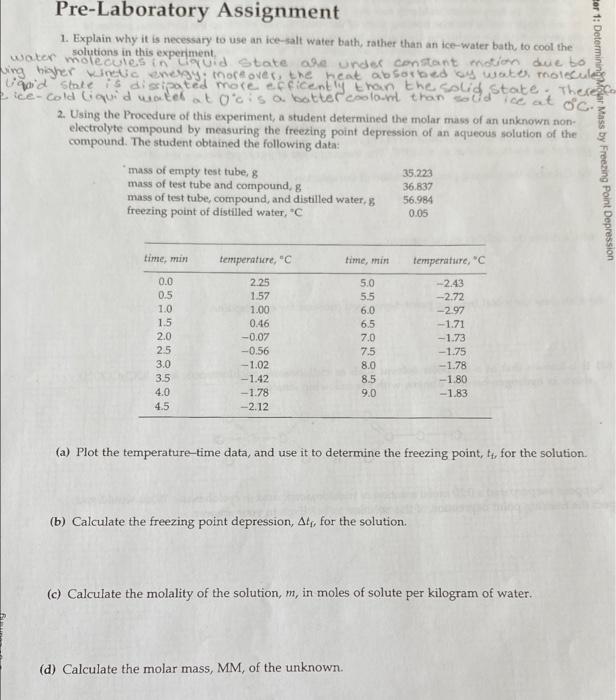
Pre-Laboratory Assignment 1. Explain why it is necessary to use an ice-salt water bath, rather than an ice-water bath, to cool the solutions in this experiment, \\\\\ molecules inuid State ale under constant mietin due to wie bisher witic energy, moreover, the heat absorbed wato, Toolecules and state is dissipated more ocecently than the Solid State. There ice-cold Guidweek atocis a better color than sodice at oc. 2. Using the Procedure of this experiment, a student determined the molar mass of an unknown non electrolyte compound by measuring the freezing point depression of an aqueous solution of the compound. The student obtained the following data: mass of empty test tube, 8 35.223 mass of test tube and compound, g mass of test tube, compound, and distilled water, 56.984 freezing point of distilled water, *C Per 1: Determining Mass by Freezing Point Depression 36.837 0.05 time, min temperature, time, min temperature, n 0.0 0.5 1.0 1.5 2.0 25 3.0 3.5 4.0 4.5 U18 USGSGES 2.25 1.57 1.00 0.46 -0.07 -0.56 - 1.02 -1.42 -1.78 -2.12 DONN06GOO 5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 -2.43 -2.72 -2.97 -1.71 -1.73 -1.75 -1.78 -1.80 -1.83 obu (a) Plot the temperature-time data, and use it to determine the freezing point, tt, for the solution. (b) Calculate the freezing point depression, At, for the solution (c) Calculate the molality of the solution, m, in moles of solute per kilogram of water. (d) Calculate the molar mass, MM, of the unknown. 3. The temperature-time data in Assignment 2 indicates that after the unknown solution super-cool to-297 Cits temperature suddenly rose to -1.71C, and then gradually drifted downward again. In order to determine the true freezing point of the solution, you extended the horizontal portion of the curve until it intersected the first part of the curve (see Figure 2). The intersection corresponded to the true freezing point of the solution. If you used -1.71C as the freezing point of the solution, Instead of -1.61 "Chow would it affect your calculated molar mass for the unknown? Chapter 1: Determining Molar Mass by Freezing Point Depression 4. Cyclohexane (CH2) can be used as a solvent for freezing point depression measurements. When 1.07 g of naphthalene (CH) (MM = 128.17 g/mol) is mixed with 51.29 g of cyclohexane, the freezing point of the resulting solution is 3.29C. The freezing point of pure cyclohexane is 6.54C. (a) Calculate the freezing point depression of the solution. (b) Calculate the molality of the solution () Calculate the molal freezing point depression constant for cyclohexane. (a) It is known that in a nonpolar solvent such as cyclohexane, trichloroacetic acid (CCI,COOH) exists as hydrogen-bonded dimers, with the following structure: O---H-0 CI CK C-C- CI CI O-H----O CI Suppose we prepared a 0.20 molal solution of trichloroacetic acid in cyclohexane. What would you expect the freezing point depression of this solution to be? Explain your