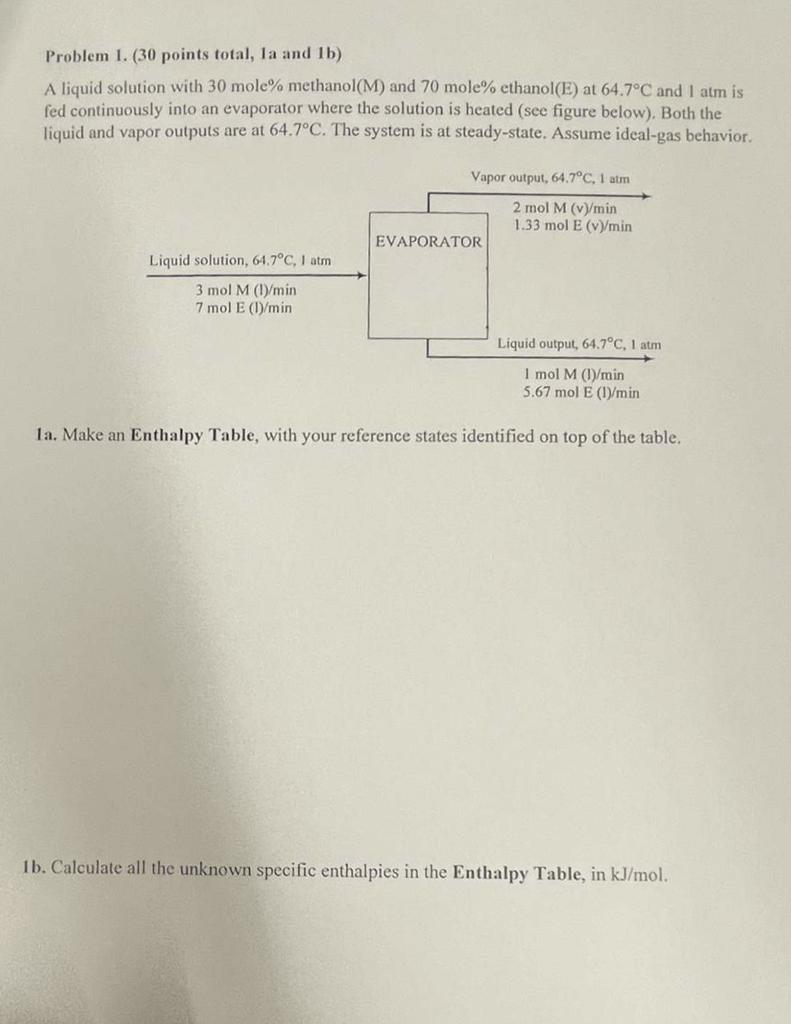
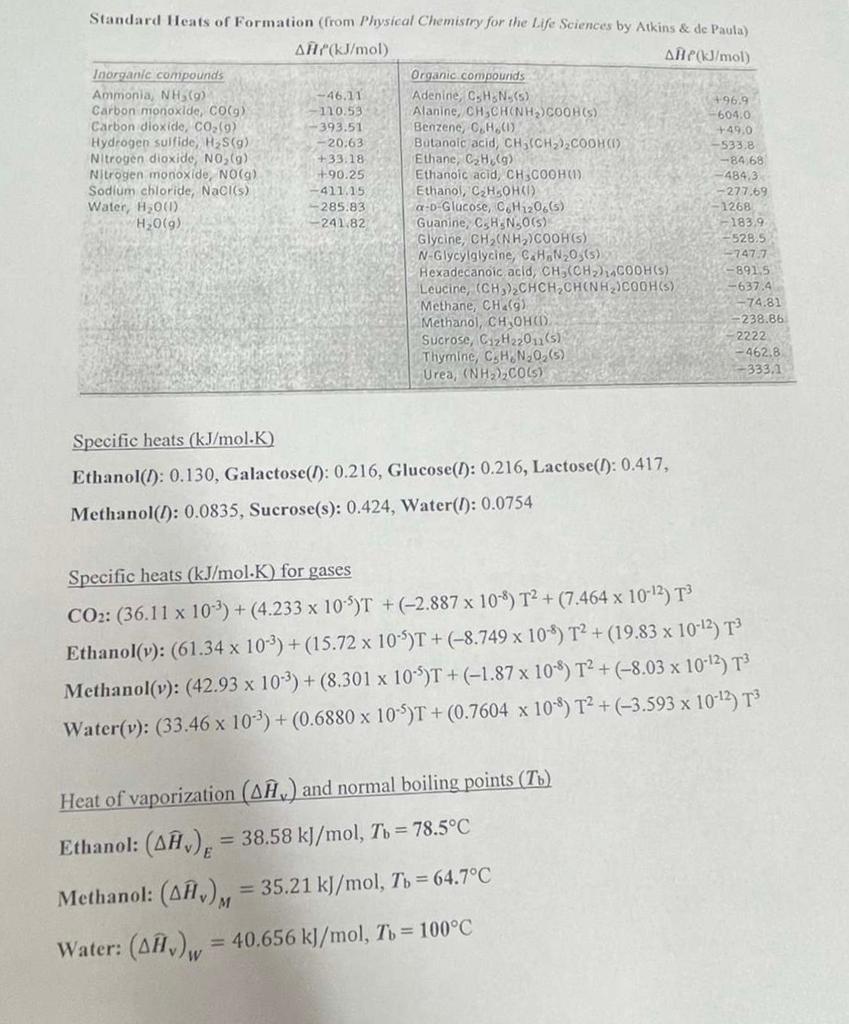
Problem 1. (30 points total, la and lb) A liquid solution with 30 mole% methanol(M) and 70 mole% ethanol(E) at 64.7C and I atm is fed continuously into an evaporator where the solution is heated (see figure below). Both the liquid and vapor outputs are at 64.7C. The system is at steady-state. Assume ideal-gas behavior. Vapor output, 64.7C, 1 atm 2 mol M (v/min 1.33 mol E (V)/min EVAPORATOR Liquid solution, 64.7C, I am 3 mol M (l/min 7 mol E (1/min Liquid output, 64.7C, 1 atm I mol M (1)/min 5.67 mol E (1)/min 1a. Make an Enthalpy Table, with your reference states identified on top of the table. 1b. Calculate all the unknown specific enthalpies in the Enthalpy Table, in kJ/mol. Standard Heats of Formation (from Physical Chemistry for the Life Sciences by Atkins & de Paula) AH(kJ/mol ANPJ/mol Inorganic compounds Organic compounds Ammonia, NH (9) -46.11 Adenine, CHN (5) +96.9 Carbon monoxide, COO) 110.53 Alanine, CH SCHONHYCOOH(S) -6040 Carbon dioxide, CO (9) -393.51 Benzene, C.H.(1) +49.0 Hydrogen sulfide, H3S(9) -- 20.63 Butanoic acid, CH(CH3)2COOHC) -533.8 Nitrogen dioxide, NO (6) +33.18 Ethane, CH (9) -84.68 Nitrogen monoxide, NO(g) +90.25 Ethanoic acid, CH3COOH(1) ana Sodium chloride, Naciis) Ethanol, C,HSOHO Water, H2001) -285. 5.83 a-b Glucose, CH 120.65) -1268 H2O(9) -241.82 - 183.9 Glycine, CH (NH)COOH(s) -528.5 N-Glycylglycine, C.H.N203(5) -747.7 Hexadecanoic acid, CH,(CH) CODH(S) -891.5 Leucine, (CH)CHCH,CHINH)COOH(S) -637.4 Methane, CH.) - 74.81 Methanol, CH OHOD -238.86 Sucrose, C2H22011 (5) Thymine, C.H.N.O.(5) - 462,8 Urea, (NH) COL) -333.1 -411.15 -4843 -277.69 anne, C.H.N.O(S) -2222 Specific heats (kJ/mol.K) Ethanol(1): 0.130, Galactose(l): 0.216, Glucose(l): 0.216, Lactose(): 0.417, Methanol(1): 0.0835, Sucrose(s): 0.424, Water(I): 0.0754 Specific heats (kJ/mol.K) for gases CO2: (36.11 x 10-3) +(4.233 x 10-5)T + (-2.887 x 10-9) T2 + (7.464 x 10-12) T3 Ethanol(y): (61.34 x 10-3) + (15.72 x 10-4)T + (-8.749 x 108) T2 + (19.83 x 10-42) T Methanol(v): (42.93 x 10-3) +(8.301 x 10-5)T + (-1.87 x 10-8) T2 + (-8.03 x 10-12) T3 Water(v): (33.46 x 10-2) + (0.6880 x 10-5)T + (0.7604 x 10-4) T2 + (-3.593 x 10-13) T3 Heat of vaporization (Aw) and normal boiling points (T6) Ethanol: (), = 38.58 kJ/mol, To = 78.5C Methanol: (AA), = 35.21 kJ/mol, Tu = 64.7C Water: (18.), = 40.656 kJ/mol, Tu = 100C Problem 1. (30 points total, la and lb) A liquid solution with 30 mole% methanol(M) and 70 mole% ethanol(E) at 64.7C and I atm is fed continuously into an evaporator where the solution is heated (see figure below). Both the liquid and vapor outputs are at 64.7C. The system is at steady-state. Assume ideal-gas behavior. Vapor output, 64.7C, 1 atm 2 mol M (v/min 1.33 mol E (V)/min EVAPORATOR Liquid solution, 64.7C, I am 3 mol M (l/min 7 mol E (1/min Liquid output, 64.7C, 1 atm I mol M (1)/min 5.67 mol E (1)/min 1a. Make an Enthalpy Table, with your reference states identified on top of the table. 1b. Calculate all the unknown specific enthalpies in the Enthalpy Table, in kJ/mol. Standard Heats of Formation (from Physical Chemistry for the Life Sciences by Atkins & de Paula) AH(kJ/mol ANPJ/mol Inorganic compounds Organic compounds Ammonia, NH (9) -46.11 Adenine, CHN (5) +96.9 Carbon monoxide, COO) 110.53 Alanine, CH SCHONHYCOOH(S) -6040 Carbon dioxide, CO (9) -393.51 Benzene, C.H.(1) +49.0 Hydrogen sulfide, H3S(9) -- 20.63 Butanoic acid, CH(CH3)2COOHC) -533.8 Nitrogen dioxide, NO (6) +33.18 Ethane, CH (9) -84.68 Nitrogen monoxide, NO(g) +90.25 Ethanoic acid, CH3COOH(1) ana Sodium chloride, Naciis) Ethanol, C,HSOHO Water, H2001) -285. 5.83 a-b Glucose, CH 120.65) -1268 H2O(9) -241.82 - 183.9 Glycine, CH (NH)COOH(s) -528.5 N-Glycylglycine, C.H.N203(5) -747.7 Hexadecanoic acid, CH,(CH) CODH(S) -891.5 Leucine, (CH)CHCH,CHINH)COOH(S) -637.4 Methane, CH.) - 74.81 Methanol, CH OHOD -238.86 Sucrose, C2H22011 (5) Thymine, C.H.N.O.(5) - 462,8 Urea, (NH) COL) -333.1 -411.15 -4843 -277.69 anne, C.H.N.O(S) -2222 Specific heats (kJ/mol.K) Ethanol(1): 0.130, Galactose(l): 0.216, Glucose(l): 0.216, Lactose(): 0.417, Methanol(1): 0.0835, Sucrose(s): 0.424, Water(I): 0.0754 Specific heats (kJ/mol.K) for gases CO2: (36.11 x 10-3) +(4.233 x 10-5)T + (-2.887 x 10-9) T2 + (7.464 x 10-12) T3 Ethanol(y): (61.34 x 10-3) + (15.72 x 10-4)T + (-8.749 x 108) T2 + (19.83 x 10-42) T Methanol(v): (42.93 x 10-3) +(8.301 x 10-5)T + (-1.87 x 10-8) T2 + (-8.03 x 10-12) T3 Water(v): (33.46 x 10-2) + (0.6880 x 10-5)T + (0.7604 x 10-4) T2 + (-3.593 x 10-13) T3 Heat of vaporization (Aw) and normal boiling points (T6) Ethanol: (), = 38.58 kJ/mol, To = 78.5C Methanol: (AA), = 35.21 kJ/mol, Tu = 64.7C Water: (18.), = 40.656 kJ/mol, Tu = 100C








