Question
Problem 1 (40/100): Calcium bicarbonate can decompose at sufficiently high temperature according to the following equation: Ca(HCO3)2(s) CaCO3(s) + H2O(g) + CO2(g) If pure
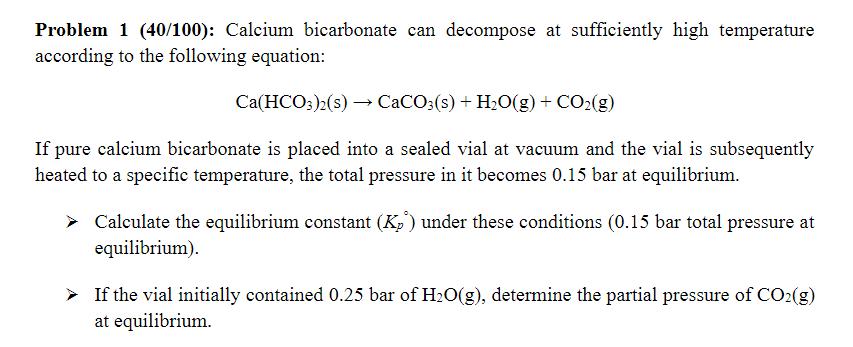
Problem 1 (40/100): Calcium bicarbonate can decompose at sufficiently high temperature according to the following equation: Ca(HCO3)2(s) CaCO3(s) + H2O(g) + CO2(g) If pure calcium bicarbonate is placed into a sealed vial at vacuum and the vial is subsequently heated to a specific temperature, the total pressure in it becomes 0.15 bar at equilibrium. Calculate the equilibrium constant (K) under these conditions (0.15 bar total pressure at equilibrium). If the vial initially contained 0.25 bar of H2O(g), determine the partial pressure of CO2(g) at equilibrium.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
4th edition
978-1118431221, 9781119192138, 1118431227, 1119192137, 978-1119498759
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



