Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. A vanadium redox flow battery involves the following electrochemical half-reactions: 3+ + e V+ E = -0.26 V E = 1.0 V (VO2)+
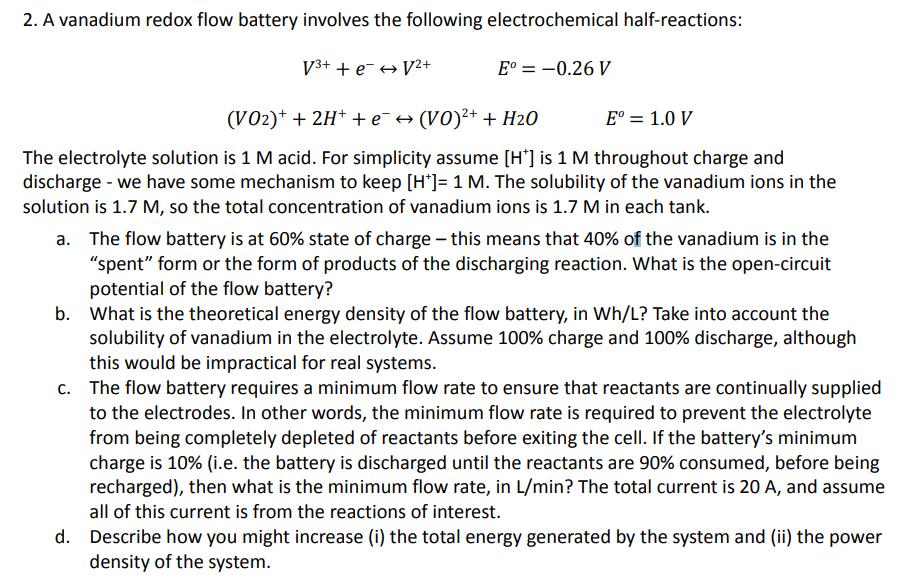
2. A vanadium redox flow battery involves the following electrochemical half-reactions: 3+ + e V+ E = -0.26 V E = 1.0 V (VO2)+ + 2H + e (VO)+ + H2O The electrolyte solution is 1 M acid. For simplicity assume [H] is 1 M throughout charge and discharge - we have some mechanism to keep [H]= 1 M. The solubility of the vanadium ions in the solution is 1.7 M, so the total concentration of vanadium ions is 1.7 M in each tank. a. The flow battery is at 60% state of charge - this means that 40% of the vanadium is in the "spent" form or the form of products of the discharging reaction. What is the open-circuit potential of the flow battery? b. What is the theoretical energy density of the flow battery, in Wh/L? Take into account the solubility of vanadium in the electrolyte. Assume 100% charge and 100% discharge, although this would be impractical for real systems. c. The flow battery requires a minimum flow rate to ensure that reactants are continually supplied to the electrodes. In other words, the minimum flow rate is required to prevent the electrolyte from being completely depleted of reactants before exiting the cell. If the battery's minimum charge is 10% (i.e. the battery is discharged until the reactants are 90% consumed, before being recharged), then what is the minimum flow rate, in L/min? The total current is 20 A, and assume all of this current is from the reactions of interest. d. Describe how you might increase (i) the total energy generated by the system and (ii) the power density of the system.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


