Question: Problem #1 A ternary mixture of hexane (A), heptane (B) and octane (C) is to be flash vaporized continuously at 1 atm pressure. The
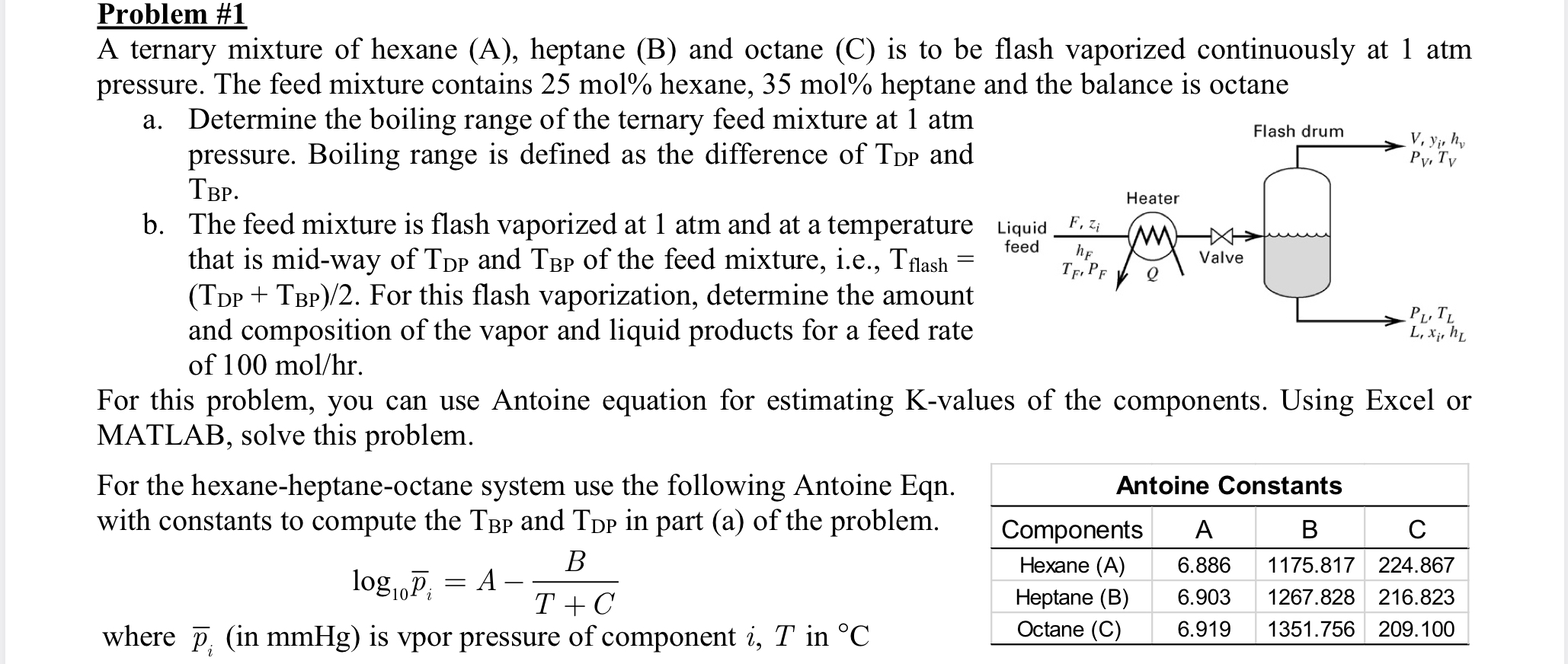
Problem #1 A ternary mixture of hexane (A), heptane (B) and octane (C) is to be flash vaporized continuously at 1 atm pressure. The feed mixture contains 25 mol% hexane, 35 mol% heptane and the balance is octane a. Determine the boiling range of the ternary feed mixture at 1 atm pressure. Boiling range is defined as the difference of TDP and TBP. b. The feed mixture is flash vaporized at 1 atm and at a temperature Liquid F, Zi that is mid-way of TDP and TBP of the feed mixture, i.e., Tflash= feed hF (TDP + TBP)/2. For this flash vaporization, determine the amount and composition of the vapor and liquid products for a feed rate of 100 mol/hr. For the hexane-heptane-octane system use the following Antoine Eqn. with constants to compute the T and Tp in part (a) of the problem. B log0Pi T + C where p (in mmHg) is vpor pressure of component i, T in 2 = TF, PF A Heater M For this problem, you can use Antoine equation for estimating K-values of the components. Using Excel or MATLAB, solve this problem. Valve Flash drum Components Hexane (A) Heptane (B) Octane (C) Antoine Constants V, yj, hv Py, Ty PL, TL L, Xj, hL A B C 6.886 1175.817 224.867 6.903 1267.828 216.823 6.919 1351.756 209.100
Step by Step Solution
3.44 Rating (163 Votes )
There are 3 Steps involved in it
The MATrix LABoratory programming language and interactive environment is intended for numerical computations data analysis and visualization It excels at handling matrices and arrays it is widely use... View full answer

Get step-by-step solutions from verified subject matter experts


