Answered step by step
Verified Expert Solution
Question
1 Approved Answer
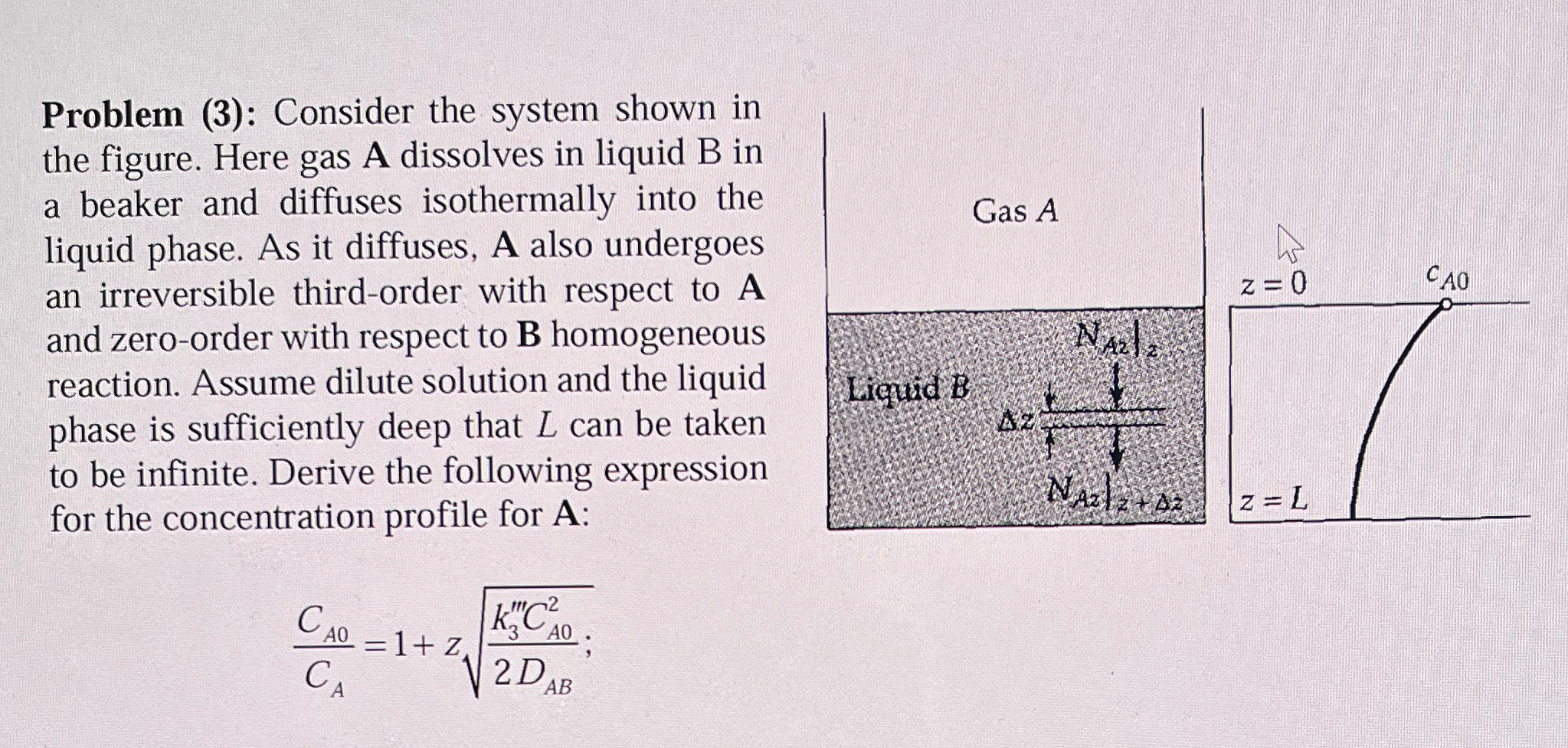
Problem ( 3 ) : Consider the system shown in the figure. Here gas A dissolves in liquid B in a beaker and diffuses isothermally
Problem : Consider the system shown in the figure. Here gas A dissolves in liquid B in a beaker and diffuses isothermally into the liquid phase. As it diffuses, A also undergoes an irreversible thirdorder with respect to A and zeroorder with respect to homogeneous reaction. Assume dilute solution and the liquid phase is sufficiently deep that can be taken to be infinite. Derive the following expression for the concentration profile for :
;
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


