Answered step by step
Verified Expert Solution
Question
1 Approved Answer
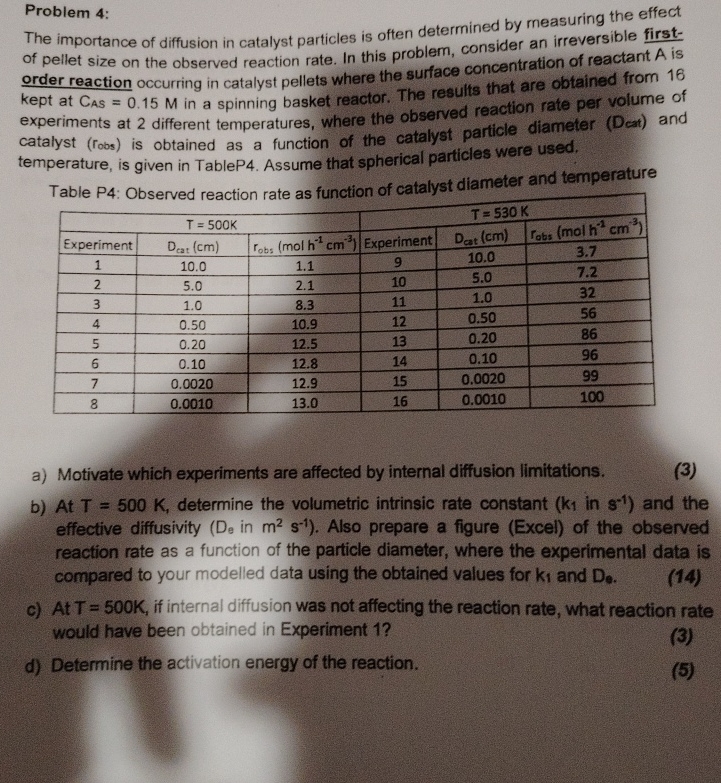
Problem 4 : The importance of diffusion in catalyst particles is often determined by measuring the effect of pellet size on the observed reaction rate.
Problem :
The importance of diffusion in catalyst particles is often determined by measuring the effect of pellet size on the observed reaction rate. In this problem, consider an irreversible firstorder reaction occurring in catalyst pellets where the surface concentration of reactant is kept at in a spinning basket reactor. The results that are obtained from experiments at different temperatures, where the observed reaction rate per volume of catalyst robs is obtained as a function of the catalyst particle diameter Dcat and temperature, is given in TableP Assume that spherical particles were used.
Table P: Observed reaction rate as function of catalyst diameter and temperature
table
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


