Answered step by step
Verified Expert Solution
Question
1 Approved Answer
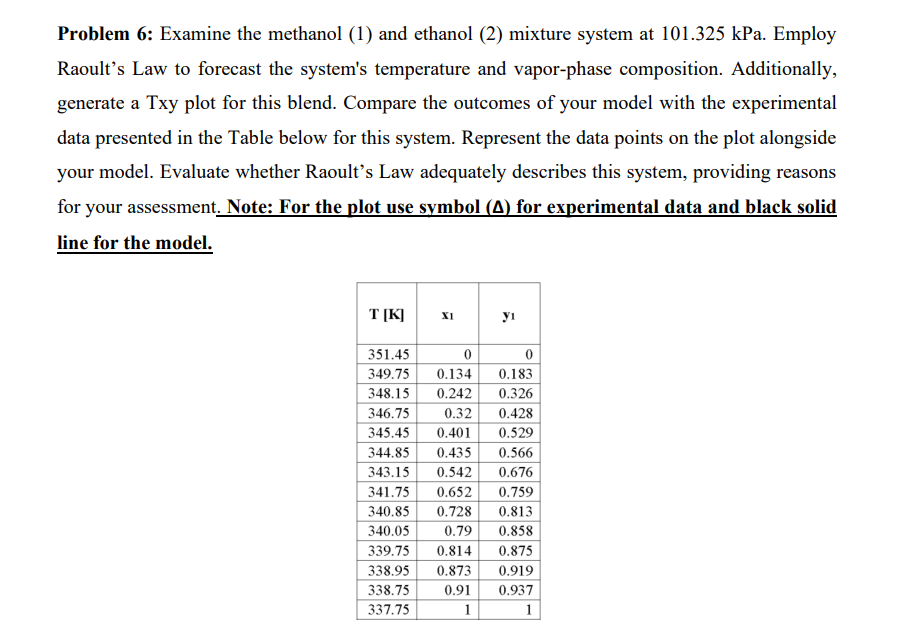
Problem 6 : Examine the methanol ( 1 ) and ethanol ( 2 ) mixture system at 1 0 1 . 3 2 5 kPa.
Problem : Examine the methanol and ethanol mixture system at kPa. Employ
Raoult's Law to forecast the system's temperature and vaporphase composition. Additionally,
generate a Txy plot for this blend. Compare the outcomes of your model with the experimental
data presented in the Table below for this system. Represent the data points on the plot alongside
your model. Evaluate whether Raoult's Law adequately describes this system, providing reasons
for your assessment. Note: For the plot use symbol for experimental data and black solid
line for the model.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


