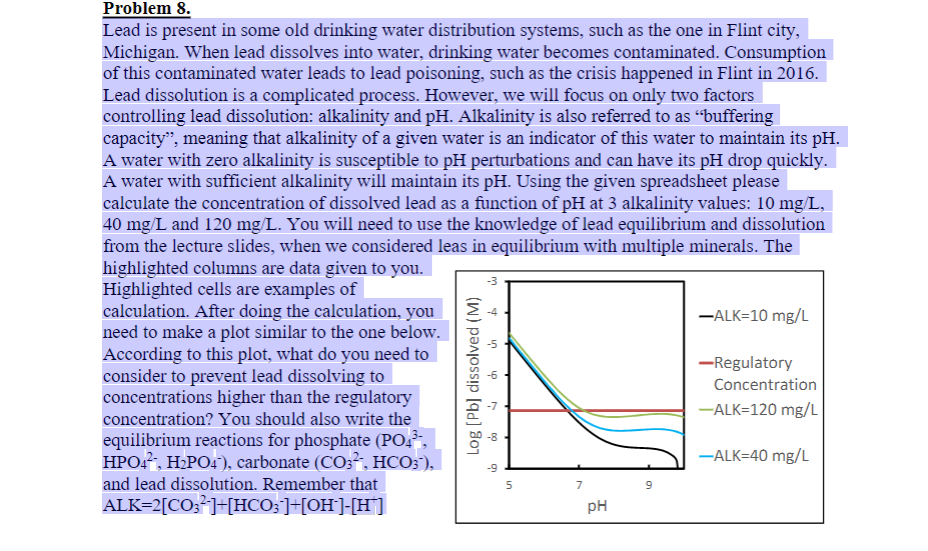
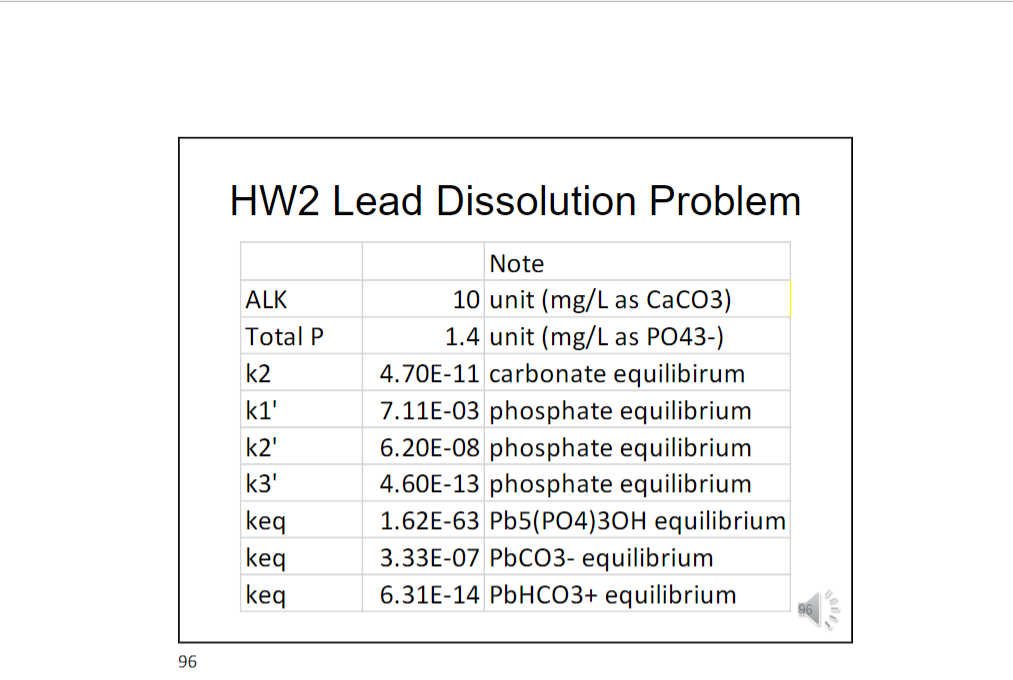
Problem 8. Lead is present in some old drinking water distribution systems, such as the one in Flint city, Michigan. When lead dissolves into water, drinking water becomes contaminated. Consumption of this contaminated water leads to lead poisoning, such as the crisis happened in Flint in 2016. Lead dissolution is a complicated process. However, we will focus on only two factors controlling lead dissolution: alkalinity and pH. Alkalinity is also referred to as buffering capacity", meaning that alkalinity of a given water is an indicator of this water to maintain its pH. A water with zero alkalinity is susceptible to pH perturbations and can have its pH drop quickly. A water with sufficient alkalinity will maintain its pH. Using the given spreadsheet please calculate the concentration of dissolved lead as a function of pH at 3 alkalinity values: 10 mg/L, 40 mg/L and 120 mg/L. You will need to use the knowledge of lead equilibrium and dissolution from the lecture slides, when we considered leas in equilibrium with multiple minerals. The highlighted columns are data given to you. Highlighted cells are examples of calculation. After doing the calculation, you -ALK=10 mg/L need to make a plot similar to the one below. According to this plot, what do you need to -Regulatory consider to prevent lead dissolving to Concentration concentrations higher than the regulatory concentration? You should also write the -ALK=120 mg/L equilibrium reactions for phosphate (POA, HPO4, H2PO4), carbonate (CO3, HCO3), -ALK=40 mg/L and lead dissolution. Remember that ALK=2(CO32-]+[HCO3-]+[OH-]-[H*] pH -3 Log (Pb] dissolved (M) do 5 -5 -9 5 7 9 HW2 Lead Dissolution Problem ALK Total P k2 ki' k2' k3' keq keq Note 10 unit (mg/L as CaCO3) 1.4 unit (mg/L as PO43-) 4.70E-11 carbonate equilibirum 7.11E-03 phosphate equilibrium 6.20E-08 phosphate equilibrium 4.60E-13 phosphate equilibrium 1.62E-63 Pb5(PO4)30H equilibrium 3.33E-07 PbCO3- equilibrium 6.31E-14 PbHCO3+ equilibrium keq 96 Problem 8. Lead is present in some old drinking water distribution systems, such as the one in Flint city, Michigan. When lead dissolves into water, drinking water becomes contaminated. Consumption of this contaminated water leads to lead poisoning, such as the crisis happened in Flint in 2016. Lead dissolution is a complicated process. However, we will focus on only two factors controlling lead dissolution: alkalinity and pH. Alkalinity is also referred to as buffering capacity", meaning that alkalinity of a given water is an indicator of this water to maintain its pH. A water with zero alkalinity is susceptible to pH perturbations and can have its pH drop quickly. A water with sufficient alkalinity will maintain its pH. Using the given spreadsheet please calculate the concentration of dissolved lead as a function of pH at 3 alkalinity values: 10 mg/L, 40 mg/L and 120 mg/L. You will need to use the knowledge of lead equilibrium and dissolution from the lecture slides, when we considered leas in equilibrium with multiple minerals. The highlighted columns are data given to you. Highlighted cells are examples of calculation. After doing the calculation, you -ALK=10 mg/L need to make a plot similar to the one below. According to this plot, what do you need to -Regulatory consider to prevent lead dissolving to Concentration concentrations higher than the regulatory concentration? You should also write the -ALK=120 mg/L equilibrium reactions for phosphate (POA, HPO4, H2PO4), carbonate (CO3, HCO3), -ALK=40 mg/L and lead dissolution. Remember that ALK=2(CO32-]+[HCO3-]+[OH-]-[H*] pH -3 Log (Pb] dissolved (M) do 5 -5 -9 5 7 9 HW2 Lead Dissolution Problem ALK Total P k2 ki' k2' k3' keq keq Note 10 unit (mg/L as CaCO3) 1.4 unit (mg/L as PO43-) 4.70E-11 carbonate equilibirum 7.11E-03 phosphate equilibrium 6.20E-08 phosphate equilibrium 4.60E-13 phosphate equilibrium 1.62E-63 Pb5(PO4)30H equilibrium 3.33E-07 PbCO3- equilibrium 6.31E-14 PbHCO3+ equilibrium keq 96








