Answered step by step
Verified Expert Solution
Question
1 Approved Answer
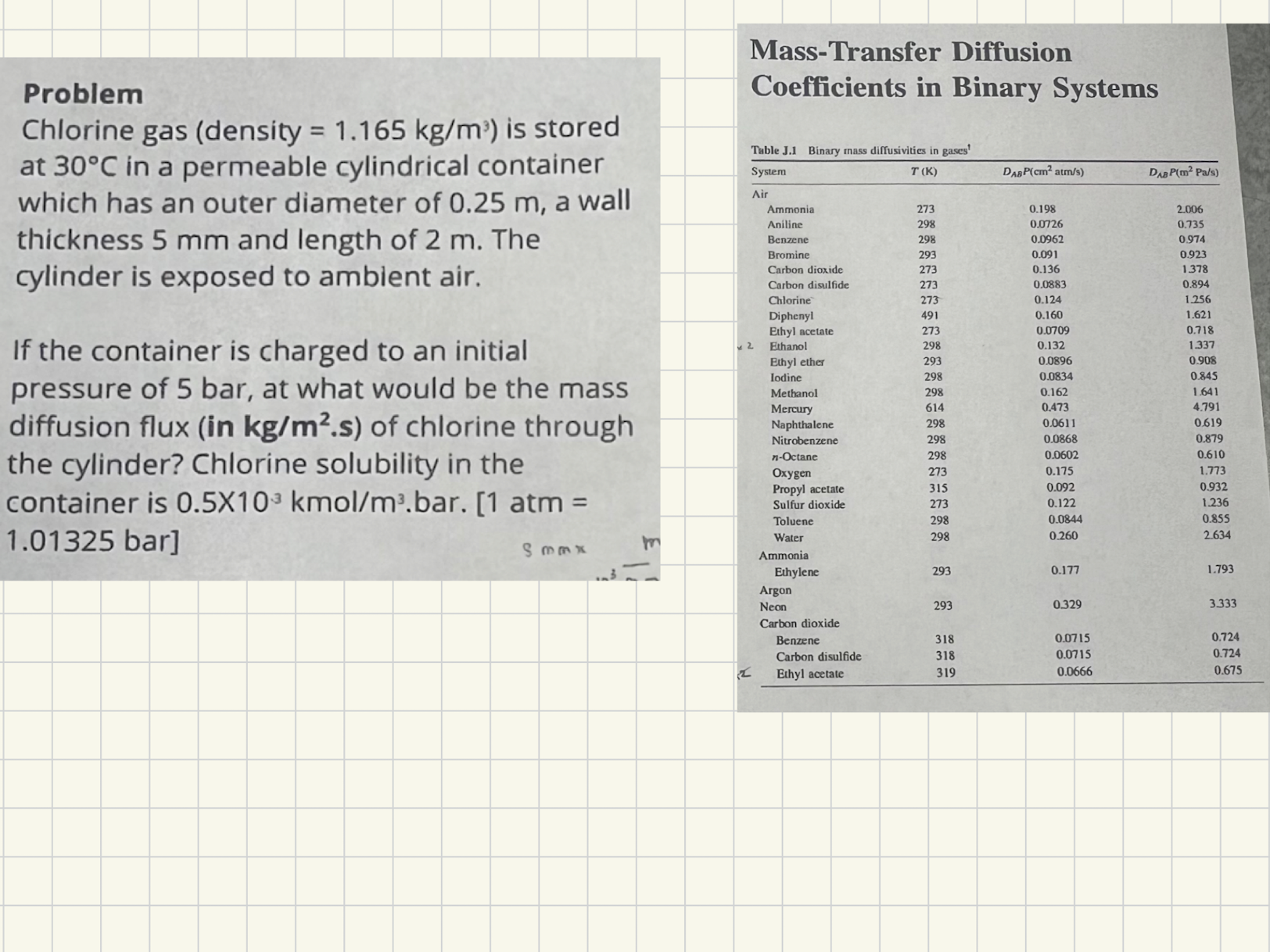
Problem Chlorine gas ( density = 1 . 1 6 5 k g m 3 ) is stored at 3 0 C in a permeable
Problem
Chlorine gas density is stored
at in a permeable cylindrical container
which has an outer diameter of a wall
thickness and length of The
cylinder is exposed to ambient air.
If the container is charged to an initial
pressure of bar at what would be the mass
diffusion flux in of chlorine through
the cylinder? Chlorine solubility in the
container is atm
bar
MassTransfer Diffusion
Coefficients in Binary Systems
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


