Answered step by step
Verified Expert Solution
Question
1 Approved Answer
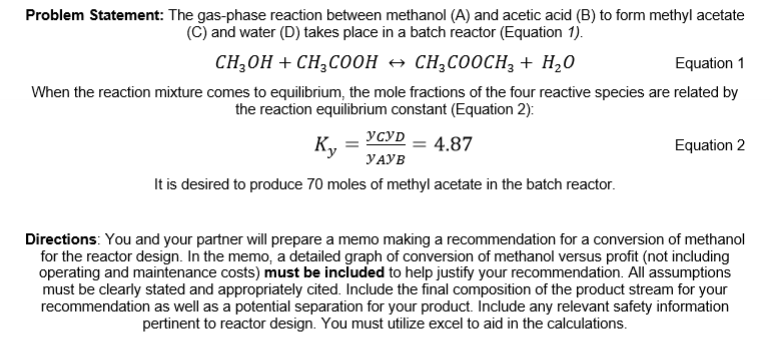
Problem Statement: The gas - phase reaction between methanol ( A ) and acetic acid ( B ) to form methyl acetate ( C )
Problem Statement: The gasphase reaction between methanol and acetic acid to form methyl acetate
C and water D takes place in a batch reactor Equation
Equation
When the reaction mixture comes to equilibrium, the mole fractions of the four reactive species are related by
the reaction equilibrium constant Equation :
Equation
It is desired to produce moles of methyl acetate in the batch reactor.
Directions: You and your partner will prepare a memo making a recommendation for a conversion of methanol
for the reactor design. In the memo, a detailed graph of conversion of methanol versus profit not including
operating and maintenance costs must be included to help justify your recommendation. All assumptions
must be clearly stated and appropriately cited. Include the final composition of the product stream for your
recommendation as well as a potential separation for your product. Include any relevant safety information
pertinent to reactor design. You must utilize excel to aid in the calculations.
PLEASE PROVIDE AN EXCEL SHEET, THANK YOU!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


