Question
Propane (C3H8) is burned in a combustion chamber at a flow rate of 18.50 m3/min with 35.0% excess air. If the propane and air streams
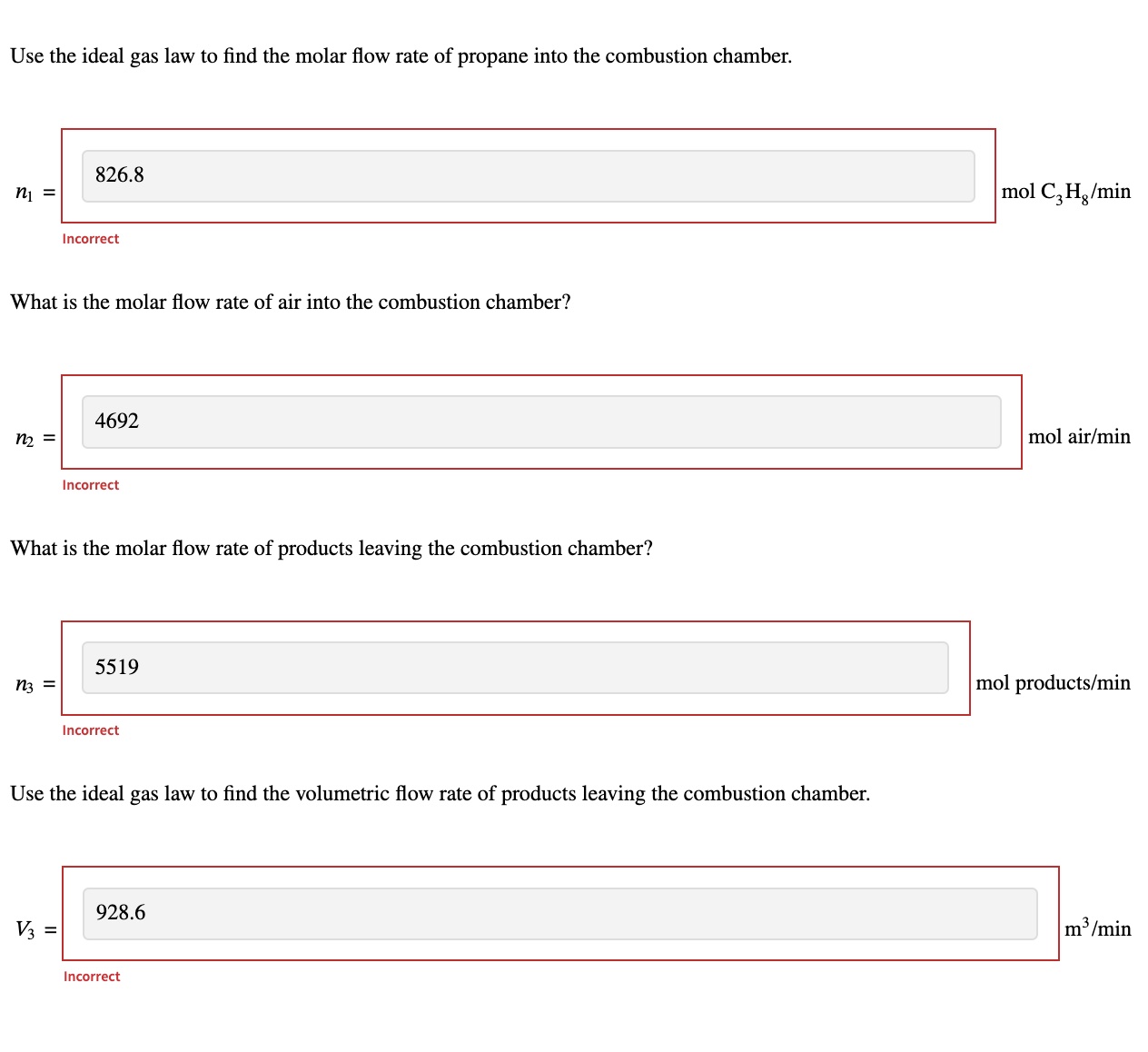
Propane (C3H8) is burned in a combustion chamber at a flow rate of 18.50 m3/min with 35.0% excess air. If the propane and air streams enter the chamber at 18.0 C and 1.15 atm (absolute), and the products leave the chamber at 270.0C and 2.40 atm (absolute), what is the volumetric flow rate of product gas from the chamber? Assume 100% conversion and ideal behavior. To solve for the flow rate, first label the process flow diagram. Label any quantities as "unknown" if they are not given or implied and "none" if the quantity is zero. Do not leave any blank spaces. Variable names are given for reference later.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


