Answered step by step
Verified Expert Solution
Question
1 Approved Answer
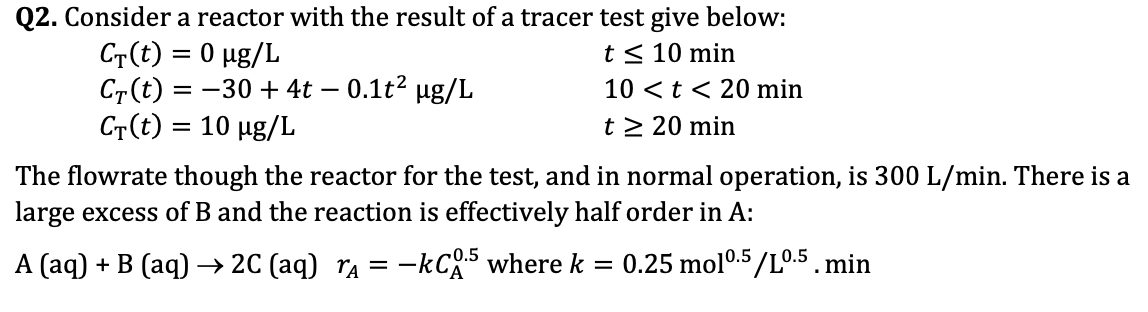
Q 2 . Consider a reactor with the result of a tracer test give below: C T ( t ) = 0 g L ,
Q Consider a reactor with the result of a tracer test give below:
min
min
The flowrate though the reactor for the test, and in normal operation, is There is a
large excess of and the reaction is effectively half order in :
where
b What is the volume of the reactor in L Integrations to be completed step by step by hand ie dont integrate numerically!
I Know that i have to use the equation dNAdt rAV but i am unsure how to derive an equation for volume without using numerical values.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


