Answered step by step
Verified Expert Solution
Question
1 Approved Answer
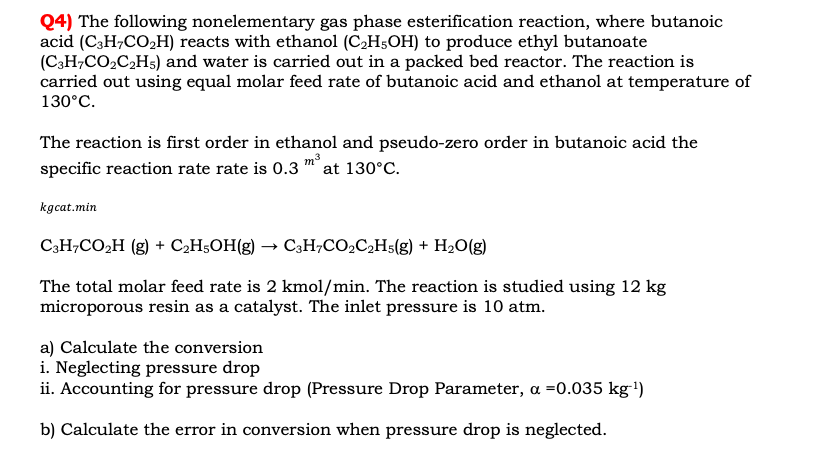
Q 4 ) The following nonelementary gas phase esterification reaction, where butanoic acid ( C 3 H 7 C O 2 H ) reacts with
Q The following nonelementary gas phase esterification reaction, where butanoic
acid reacts with ethanol to produce ethyl butanoate
and water is carried out in a packed bed reactor. The reaction is
carried out using equal molar feed rate of butanoic acid and ethanol at temperature of
The reaction is first order in ethanol and pseudozero order in butanoic acid the
specific reaction rate rate is at
kgcat.min
The total molar feed rate is kmo The reaction is studied using
microporous resin as a catalyst. The inlet pressure is atm.
a Calculate the conversion
i Neglecting pressure drop
ii Accounting for pressure drop Pressure Drop Parameter,
b Calculate the error in conversion when pressure drop is neglected.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


