Question
Q1. The formal potential (E 0 ) of the Fe3+/Fe2+ redox pair was measured in three kinds of acids with pH=0, and the following values
Q1.
The formal potential (E0) of the Fe3+/Fe2+ redox pair was measured in three kinds of acids with pH=0, and the following values were obtained. In which acid does the Fe3+ ion have the strongest oxidation power? In which acid does the Fe3+ ion form the strongest complex?
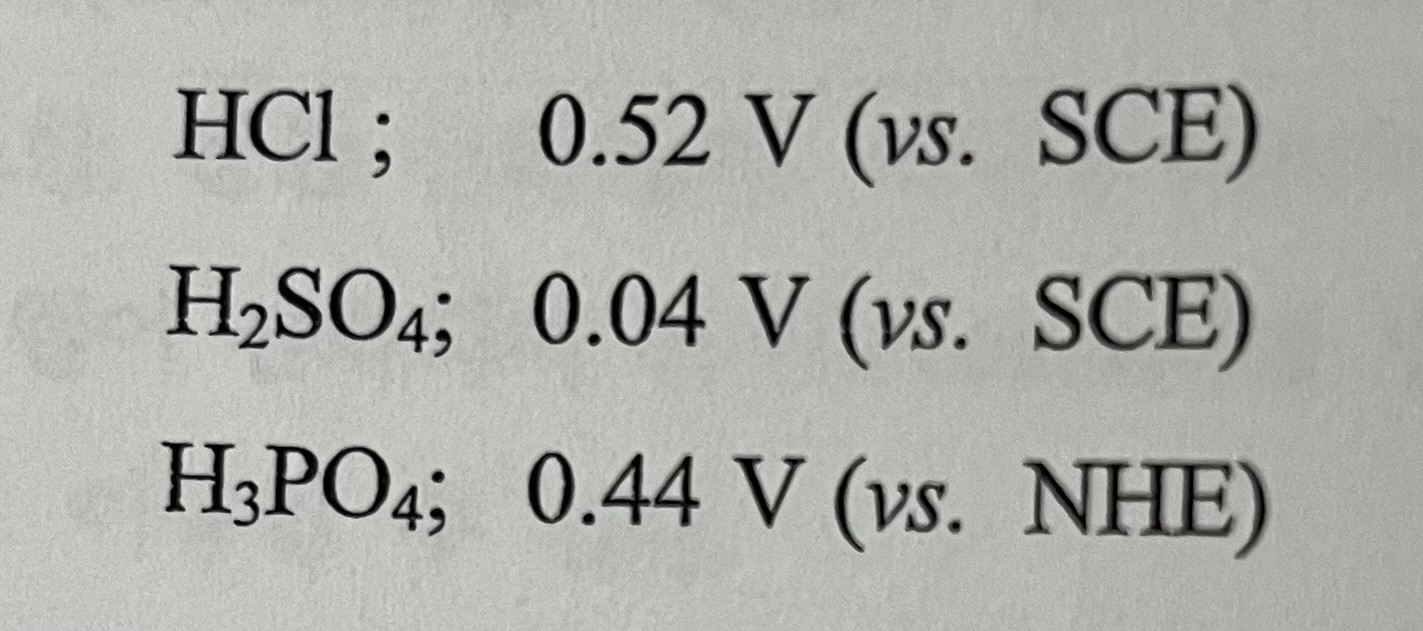
Q2.
The voltage between the two Cd electrodes in the following electrochemical cell was measured and it was -0.41 V at 25 degrees. If Cd2+ ions and CN-ions form Cd(CN)42-, what is the equilibrium constant(Kf ,formation constant) of complex formation? Ignore the inter-liquid contact potential between the separators.
Cd l Cd2+(0.01M) ll Cd2+ (0.01M), CN-(0.1M) / Cd
HCl;0.52V(vs. SCE) H2SO4;0.04V (vs. SCE) H3PO4;0.44V (vs. NHE)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


