Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q2- Iron blast furnace smelting composition ore (Fe3O4 70%, Fe2O3 5%, SiO2 10%, P2O5 1%, MnO2 4%, H2O 10%). It produces daily 900 metric tons
Q2- Iron blast furnace smelting composition ore (Fe3O4 70%, Fe2O3 5%, SiO2 10%, P2O5 1%, MnO2 4%, H2O 10%). It produces daily 900 metric tons of iron ore. Sufficient flux is used to give slag containing 24% CaO. Flux is pure limestone. 0.48 tons of coke are used per ton of ore. Coke is 86% carbon, 9% SiO2, 5% water. All the charged P, half of the Mn, and one-fifth of the Si are reduced and go into the pig iron, which by analysis also contains 4% C. 88% of the coke carbon is burned in the tweezers. Do not assume there is iron in the slag. Find:
a) The percentage composition of cast iron
b) The daily weight of molten ore
c) The flow weight used per day
d) the percentage composition of flue gases
Pure FeO is reduced to Fe, and all
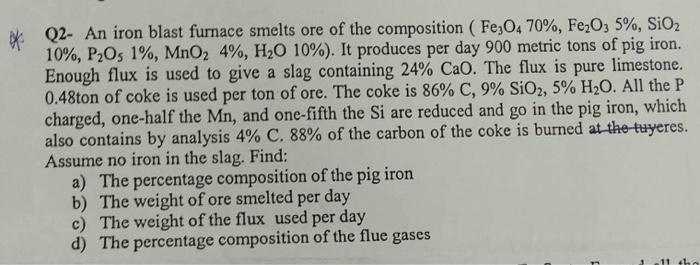
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


