Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q3: Ammonia (A) diffuses through a stagnant layer of air (B), 1.0 cm thickness, at 25C and 1.0 atm total pressure. The partial pressures of
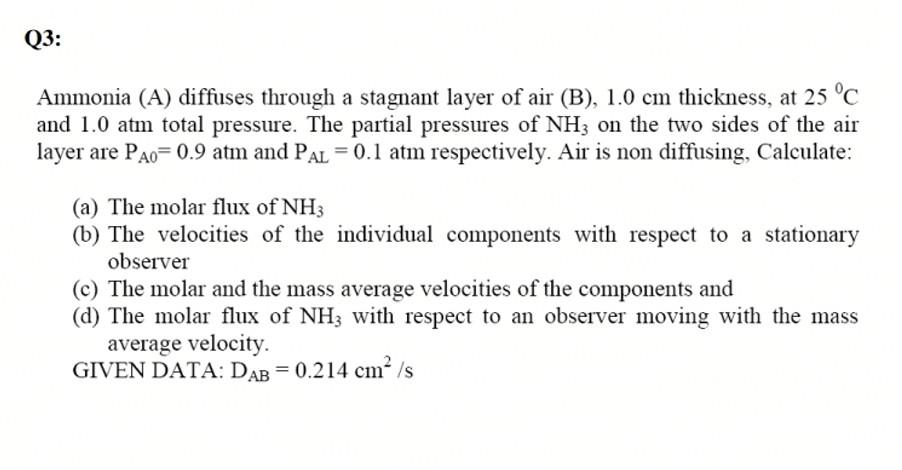
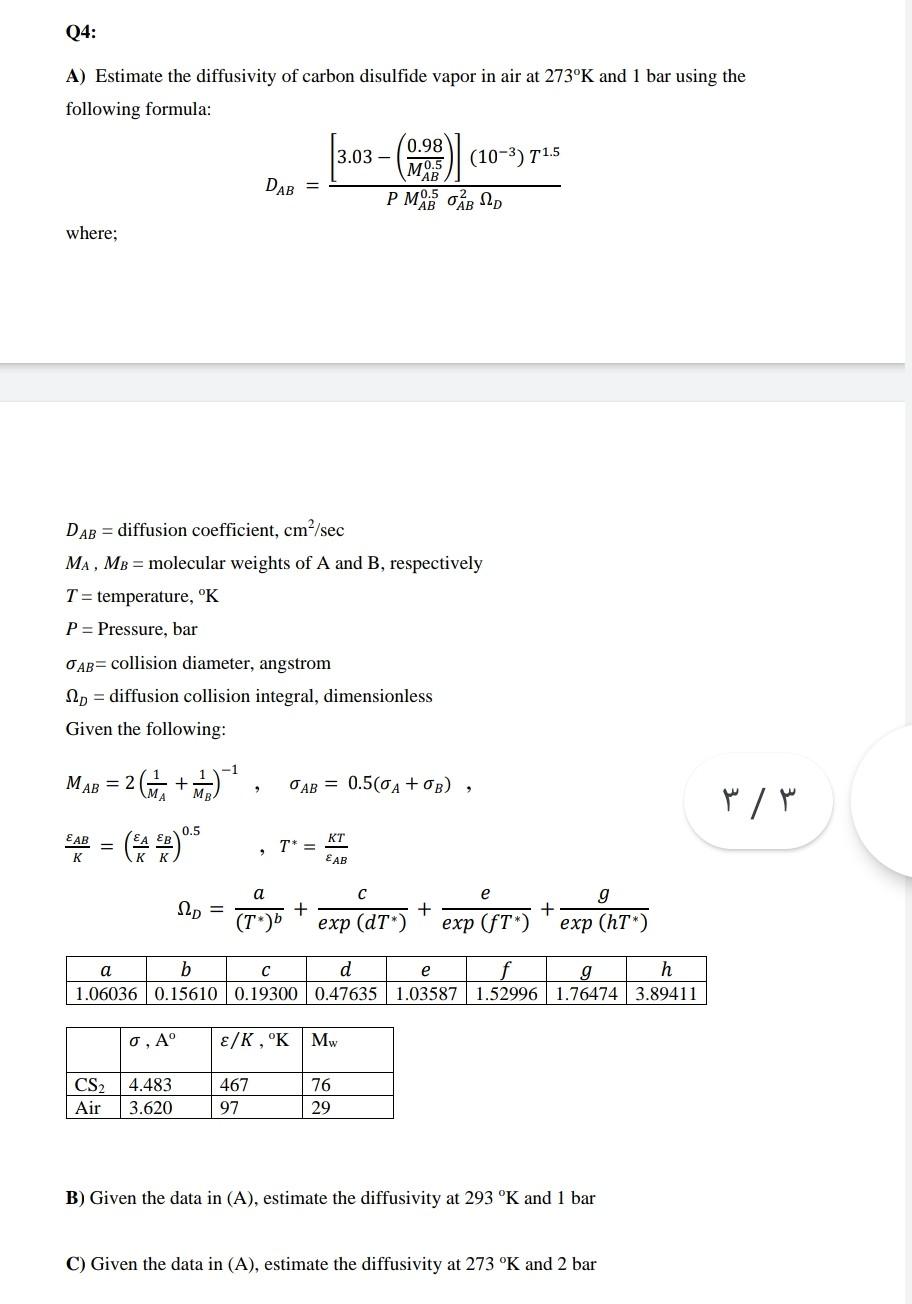
Q3: Ammonia (A) diffuses through a stagnant layer of air (B), 1.0 cm thickness, at 25C and 1.0 atm total pressure. The partial pressures of NH3 on the two sides of the air layer are P^o= 0.9 atm and Pal = 0.1 atm respectively. Air is non diffusing, Calculate: (a) The molar flux of NH3 (b) The velocities of the individual components with respect to a stationary observer (c) The molar and the mass average velocities of the components and (d) The molar flux of NH3 with respect to an observer moving with the mass average velocity. GIVEN DATA: DAB=0.214 cm/s Q4: A) Estimate the diffusivity of carbon disulfide vapor in air at 273K and 1 bar using the following formula: 0.98 3.03 - (10-3) 11.5 M0.5 DAB PMB O B SD AB where; DAB = diffusion coefficient, cm/sec MA, MB = molecular weights of A and B, respectively T = temperature, K P= Pressure, bar AB= collision diameter, angstrom 12p = diffusion collision integral, dimensionless Given the following: -1 MAB = 2 Gia + a)? OAB = 0.50A +OB) MR / 0.5 EAB KT = T* = EAB a e 12p = (T) + + exp (DT) exp (ft") + expone ) g () a b d e g h 1.06036 0.15610 0.19300 0.47635 1.03587 1.52996 1.76474 3.89411 O, A E/K, "K MW CS2 Air 4.483 3.620 467 97 76 29 B) Given the data in (A), estimate the diffusivity at 293 K and 1 bar C) Given the data in (A), estimate the diffusivity at 273 K and 2 bar
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


