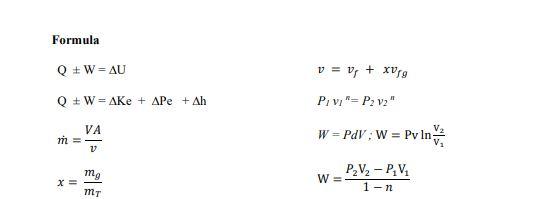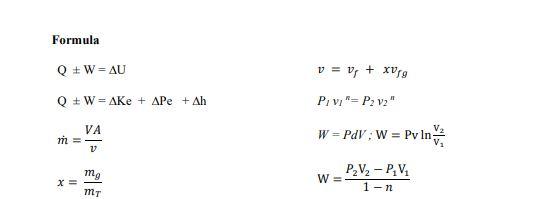
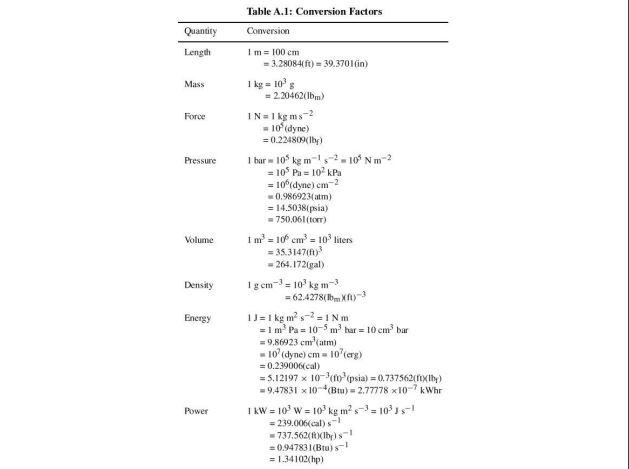
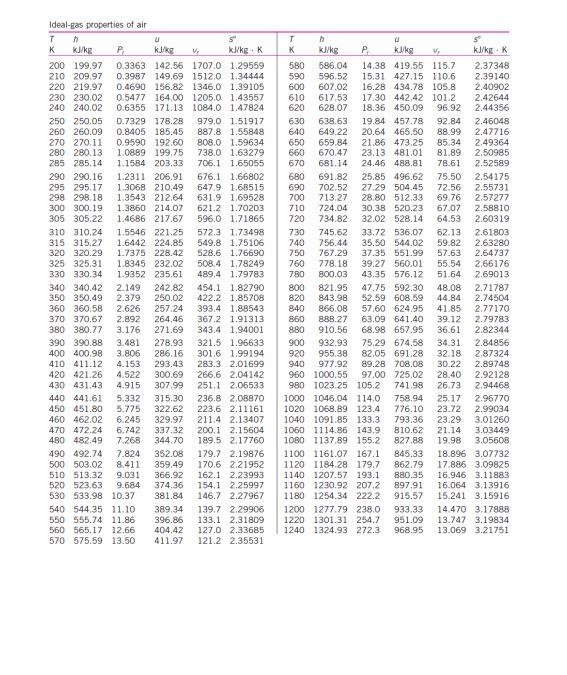
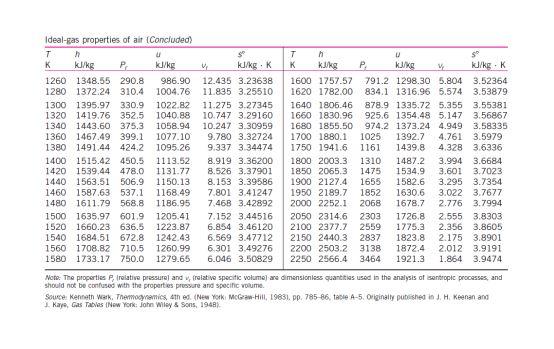
Q3 Based on the steam properties table, illustrate each of the following phases in a T-v diagram with respect to saturation lines. (a) P= 350 kPa and v=0.524 m /kg. (2 marks) (b) P= 500 kPa and T = 90 C. (2 marks) Formula Q+W=AU v = 0; + xurg Q + W = Ake + APE + Ah P/ vi"= P2 V2" VA m = 1 W = POVW = Pv In V mo PzV2 - P.V. W= 1-n X= MT Table A.1: Conversion Factors Conversion Quantity Length Mass Force Pressure Volume I m = 100 cm = 3.28084() - 39.3701 in) 1 kg = 10 = 2.20462(Ibm) IN = 1 kg m-2 -10 (dyne) = 0.224809(lb) 1 bar -10% kg m-1-2-10Nm = 10 Pa = 10 kPa = 10 dyne) cm-2 0.986923(atm) = 14.5038[psia) = 750.061(tor) 1 1m = 10 cm = 103 liters = 35.314702 - 264. 172(gal) Igem) - 10kg m-3 = 62.4278(15mA 1) = 1 kg m-2-1 Nm = 1 m. Pa = 10-5 m bar = 10 embar = 9,86023 cm'atm) -10 (dyne) cm = 10 (erg) = 0.239006(cal) = 5.12197 x 10 topsia) = 0.737562fy lly) = 9,47831 x 10-*(Btu) = 2,77778 x10-7 kWh I kW = 10' w = 10kg m? s-) = 105! = 239.006(cal)-1 - 737.562- =0.94783 (Btu)! = 1.34102(hp) Density Energy Power 4 620 660 Ideal-gas properties of air T n S KJ/kg P kJ/kg kJ/kg. K 200 199.97 0.3363 142.56 1707.0 1.29559 210 209.97 0.3987 149.69 1512.0 1.34444 220 219.97 0.4690 156.82 1346.0 1.39105 230 230.02 0.5477 164.00 1205.0 1.43557 240 240.02 0.6355 171.13 1084.0 1.47824 250 250.05 0.7329 178.28 979.0 1.51917 260 260.09 0.8405 185.45 887.8 1.55848 270 270.11 0.9590 192.60 808.0 1.59634 280 280.13 1.0889 199.75 738.0 1.63279 285 285.14 1.1584 203.33 706.1 1.65055 290 290.16 1.2311 206.91 676.1 1.66802 295 295.17 1.3068 210.49 647.9 1.68515 298 298.18 1.3543 212.64 631.9 1.69528 300 300.19 1.3860 214.07 621.2 1.70203 305 305.22 1.4686 217,67 596.0 1.71865 310 310.24 1.5546 221.25 572.3 1.73498 315 315.27 1.6442 224.85 549.8 1.75106 320 320.29 1.7375 228.42 528.6 1.76690 325 325.31 1.8345 232.02 508.4 1.78249 330 330.34 1.9352 235.61 489.4 1.79783 340 340.42 2.149 242.82 454.1 1.82790 350 350.49 2.379 250.02 422.2 1.85708 360 360.58 2.626 257.24 393.4 1.88543 370 370.67 2.892 264.46 367.2 1.91313 380 380.77 3.176 271.69 343.4 1.94001 390 390.88 3.481 278.93 321.5 1.96633 400 400.98 3.806 286.16 301.6 1.99194 410 411.12 4.153 293.43 283.3 2.01699 420 421.26 4.522 300.69 266.6 2.04142 430 431,43 4.915 307.99 251.1 2.06533 440 441.61 5.332 315.30 236.8 2.08870 450 451.80 5.775 322.62 223.6 2.11161 460 462.02 6.245 329.97 211.4 2.13407 470 472.24 6.742 337.32 200.1 2.15604 480 482.49 7.268 344.70 189.5 2.17760 490 492.74 7.824 352.08 179.7 2.19876 500 503.02 8.411 359.49 170.6 2.21952 510 513.32 9.031 366.92 162.1 2.23993 520 523.63 9.684 374.36 154.1 2.25997 530 533.98 10.37 381.84 146.7 2.27967 540 544,35 11.10 389.34 139.7 2.29905 550 555.74 11.86 396.86 133.1 2.31809 560 565.17 12.66 404,42 127.0 2.33685 570 575.59 13.50 411.97 121.2 2.35531 T h s K k/kg P kg V kl/kgK 580 586.04 14.38 419.55 115.7 2.37348 590 596.52 15.31 427.15 110.6 2.39140 600 607.02 16.28 434.78 105.8 2.40902 610 617.53 17.30 442,42 101.2 2.42644 628.07 18.36 450.09 96.92 2.44356 630 638.63 19.84 457.78 92.84 2.46048 640 649.22 20.64 465.50 88.99 2.47716 650 659.84 21.86 473.25 85.34 2.49364 670.47 23,13 481.01 81.89 2.50985 670 681.14 24.46 488.81 78.61 2.52589 680 691.82 25.85 496.62 75.50 2.54175 690 702.52 27.29 504.45 72.56 2.55731 700 713.27 28.80 512.33 69.76 2,57277 710 724.04 30.38 520.23 67.07 2.58810 720 734.82 32.02 528.14 64.53 2.60319 730 745.62 33.72 536.07 62.13 2.61803 740 756.44 35.50 544.02 59.82 2.63280 750 767.29 37.35 551.99 57.63 2.64737 760 778.18 39.27 560.01 55.54 2.66176 780 800.03 43.35 576.12 51.64 2.69013 800 821.95 47.75 592.30 48.08 2.71787 820 843.98 52.59 608.59 44.84 2.74504 840 866.08 57.60 624.95 41.85 2.77170 860 888.27 63.09 641,40 39.12 2.79783 880 910.56 68.98 657.95 36.61 2.82344 900 932.93 75.29 674.58 34.31 2.84856 920 955.38 82.05 691.28 32.18 2.87324 940 977.92 89.28 708.08 30.22 2.89748 960 1000.55 97.00 725,02 28.40 2.92128 980 1023.25 105.2 741.98 26.73 2.94468 1000 1046.04 114.0 758.94 25.17 2.96770 1020 1068.89 123.4 776.10 23.72 2.99034 1040 1091.85 133.3 793.36 23.29 3.01260 1050 1114.86 143.9 810.62 21.14 3.03449 1080 1137,89 155.2 827.88 19.98 3.05606 1100 1161.07 167.1 845,33 18.896 3.07732 1120 1184.28 179.7 862.79 17.886 3.09825 1140 1207.57 193.1 880.35 16.946 3.11883 1160 1230.92 207,2 897.91 16.064 3.13916 1180 1254.34 222.2 915.57 15.241 3.15916 1200 1277.79 238.0 933.33 14.470 3.17888 1220 1301.31 254.7 951.09 13.747 3.19834 1240 1324.93 272.3 968.95 13.069 3.21751 Ideal-gas properties of air (Concluded) T u k/kg P k/kg 1260 1348.55 290.8 986.90 1280 1372.24 310.4 1004.76 1300 1395.97 330.9 1022.82 1320 1419.76 352.5 1040.88 1340 1443.60 375.3 1058.94 1360 1467.49 399.1 1077.10 1380 1491.44 424.2 1095.26 1400 1515.42 450.5 1113.52 1420 1539.44 478.0 1131.77 1440 1563.51 506.9 1150.13 1460 1587.63 537.1 1168.49 1480 1611.79 568.8 1186.95 1500 1635.97 601.9 1205.41 1520 1660.23 636.5 1223.87 1540 1684.51 672.8 1242.43 1560 1708.82 710.5 1260.99 1580 1733.17 750.0 1279.65 se k/kg. K 12.435 3.23638 11.835 3.25510 11.275 3.27345 10.747 3.29160 10.247 3.30959 9.780 3.32724 9.337 3.34474 8.919 3.36200 8.526 3.37901 8.153 3.39586 7.801 3.41247 7.46B 3.42892 7.152 3.44516 6.854 3.46120 6.569 3.47712 6.301 3.49276 6.046 3.50829 T h K kJ/kg 1600 1757.57 1620 1782.00 1640 1806.46 1660 1830.96 1680 1855.50 1700 1880.1 1750 1941.6 1800 2003.3 1850 2055.3 1900 2127.4 1950 2189.7 2000 2252.1 2050 2314.6 2100 2377.7 2150 2440.3 2200 2503.2 2250 2566.4 U SP P. k/kg V kul/kg : K 791.2 1298.30 5.804 3.52364 834.1 1316.96 5.574 3.53879 878.9 1335.72 5.355 3.55381 925.6 1354.48 5.147 3.56867 974.2 1373.24 4.949 3.58335 1025 1392.7 4.761 3.5979 1161 1439.8 4.328 3.6336 1310 1487.2 3.994 3.6684 1475 1534.9 3.601 3.7023 1655 1582.6 3.295 3.7354 1852 1630.6 3.022 3.7677 2068 1678.7 2.776 3.7994 2303 1726.8 2.555 3.8303 2559 1775.3 2.356 3.8605 2837 1823.8 2.175 3.8901 3138 1872.4 2.012 3.9191 3464 1921.3 1.864 3.9474 Note: The properties relative pressure and relative specific volume are dimensiones quantities used in the analysis of sentropic processes, and should not be confused with the properties pressure and specific volume Source: Kenneth Walk Themoramics, es (New York: McGraw-Hill, 19831, pp. 785-86. table A-5. Originally published in JH Keenan ang 1. Kaye, Gas Tables New York: John Wiley & Sons, 1948)