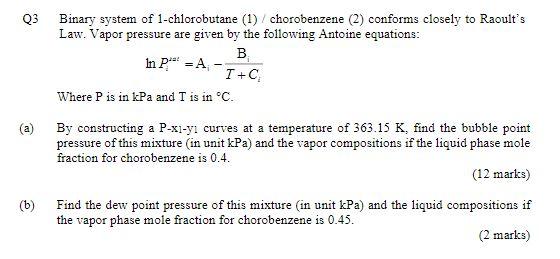
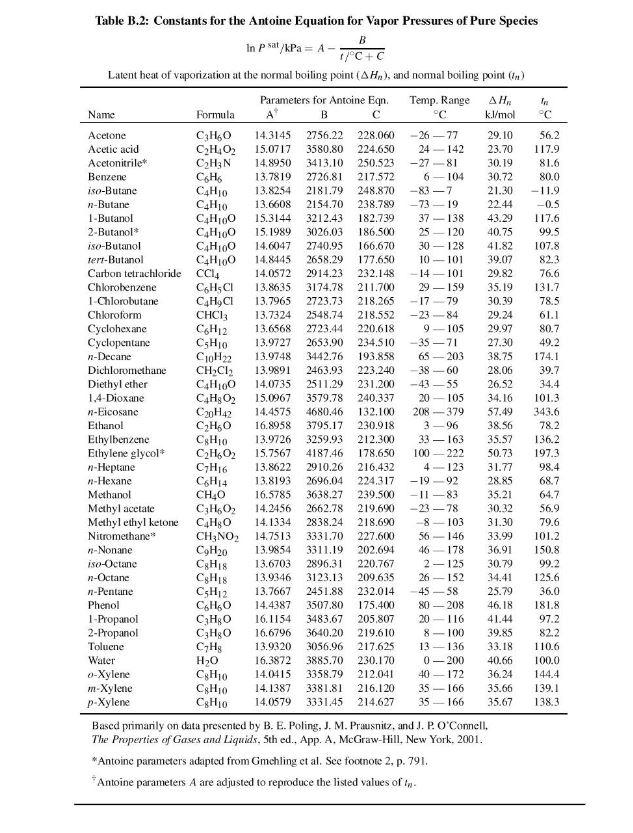
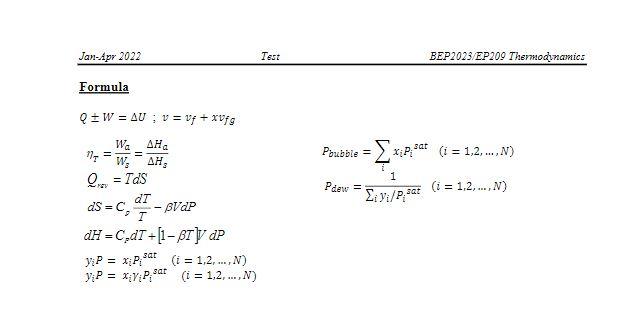
= Q3 Binary system of 1-chlorobutane (1) /chorobenzene (2) conforms closely to Raoult's Law. Vapor pressure are given by the following Antoine equations: . In P." - A I + Where P is in kPa and T is in C. (a) By constructing a P-xl-ycurves at a temperature of 363.15 K, find the bubble point pressure of this mixture in unit kPa) and the vapor compositions if the liquid phase mole fraction for chorobenzene is 0.4. (12 marks) (b) Find the dew point pressure of this mixture (in unit kPa) and the liquid compositions if the vapor phase mole fraction for chorobenzene is 0.45. (2 marks) Table B.2: Constants for the Antoine Equation for Vapor Pressures of Pure Species B In p sat/kPa = A- 1/C+C Latent heat of vaporization at the normal boiling point AH), and nomal boiling point (.) Parameters for Antoine Eqn. Temp. Range , In Name Formula B c C k.l/mol C Acetone 360 14.3145 2756.22 228.060 -26-77 29.10 56.2 Acetic acid C2H4O2 15.0717 3580.80 224.650 24 142 23.70 117.9 Acetonitrile CHEN 14.8950 3413.10 250.523 -27-81 30.19 81.6 Benzene Ca Ho 13.7819 2726.81 217.572 6-104 30.72 80.0 iso-Butane CHIO 13.8254 2181.79 248.870 -83-7 21.30 -11.9 n-Butane CHIO 13.6608 2154.70 238.789 ---73-19 22.44 -0.5 1-Butanol C4H100 15.3144 32 12.43 182.739 37 138 43.29 117.6 2-Butanol C4H100 15.1989 3026.03 186.500 25-120 40.75 99.5 iso-Butanol C4H100 14.6047 2740.95 166.670 30 128 41.82 107.8 Tert-Butanol C4H100 14.8445 2658.29 177.650 10-101 39.07 823 Carbon tetrachloride CC14 14.0572 2914.23 232.148 - 14 - 101 29.82 76.6 Chlorobenzene CHCI 13.8635 3174.78 211.700 29-159 35.19 131.7 1 Chlorobutane C4H9C1 13.7965 2723.73 218.265 -1779 30.39 78.5 Chloroform CHCl3 13.7324 2548.74 218.552 -2384 29.24 61.1 Cyclohexane CH12 13.6568 2723.44 220.618 9-105 29.97 80.7 Cyclopentane CsHio 13.9727 2653.90 234.510 -35 - 71 27.30 49.2 1-Decane COH22 13.9748 3442.76 193.858 65-203 38.75 174.1 Dichloromethane CHCl3 13.9891 2463.93 223.240 38-60 28.06 39.7 Diethyl ether C4H100 14.0735 2511.29 231.200 -43-55 26.52 34.4 1,4-Dioxane C4H2O2 15.0967 3579.78 240.337 20 105 34.16 101.3 n-Eicosane C20H42 14.4575 4680.46 132.100 208 379 57.49 343.6 Ethanol CHO 16.8958 3795.17 230.918 3-96 38.56 78.2 Ethylbenzene Cg 13.9726 3259.93 212.300 33-163 35.57 136.2 Ethylene glycol* CH.02 15.7567 4187.46 178.650 100_222 50.73 197.3 -Heptane C7H16 13.8622 2910.26 216.432 4 123 31.77 98.4 -Hexane CH14 13.8193 2696.04 224.317 -1992 28.85 68.7 Methanol CHO 16.5785 3638.27 239.500 -11-83 35.21 64.7 Methyl acetate C3H602 14.2456 2662.78 219.6%) -23 -78 30.32 56.9 Methyl ethyl ketone C&H O 14.1334 2838.24 218.690 8103 31.30 79.6 Nitromethane CH:NO2 14.7513 3331.70 227.600 56146 33.99 101.2 -Nonane C9H20 13.9854 3311.19 202.694 46178 36.91 150.8 iso-Octane 13.6703 2896.31 220.767 2-125 30.79 99.2 n-Octane CyH18 13.9346 3123.13 209.635 26-152 34.41 125.6 7-Pentane C3H12 13.7667 2451.88 232.014 45-58 25.79 36.0 Phenol CHO 14.4387 3507.80 175.400 80 - 208 46.18 181.8 1-Propanol CHO 16.1154 3483.67 205.807 20 116 41.44 97.2 2-Propanol C3H30 16.6796 3640.20 219.610 8-100 39.85 82.2 Toluene CH 13.9320 3056.96 217.625 13 136 33.18 110.6 Water HO 16.3872 3885.70 230.170 0-200 40.66 100.0 0-Xylene CHyo 14.0415 3358.79 212.041 40-172 36.24 144.4 m-Xylene CH10 14.1387 3381.81 216.120 35166 35.66 139.1 p-Xylene 14.0579 3331.45 214.627 35166 35.67 138.3 Bascd primarily on data presented by B. E. Poling, J. M. Prausnitz, and J.P. O'Connell. The Properties of Gases and Liquids, 5th ed.. App. A. McGraw-Hill, New York. 2001. *Antoinc parameters adapted from Gmchling et al. See footnote 2, p. 791. Antoine parameters A are adjusted to reproduce the listed values of In CHIS CHIO Jan-Apr 2022 Test BEP2023 EP209 Thermodynamics Formula Q+W = AU ; v = vf + xvfg WAHA 1WAH Poubble = xip,sat (1 = 1,2,...N) = Parc 1 Eivi/Paar (i = 1,2,...,N) ds = C, = Q = TDS dT - BVdP T dH=C,MT +[1- BT 4P y: P = xipat (i = 1,2, ...,N) y: P = xiyosat (i = 1,2, ...,N)









