Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Q3. Use the FIFTH digit of your student ID number and Table 3-1 to determine which material you will use for this question. For
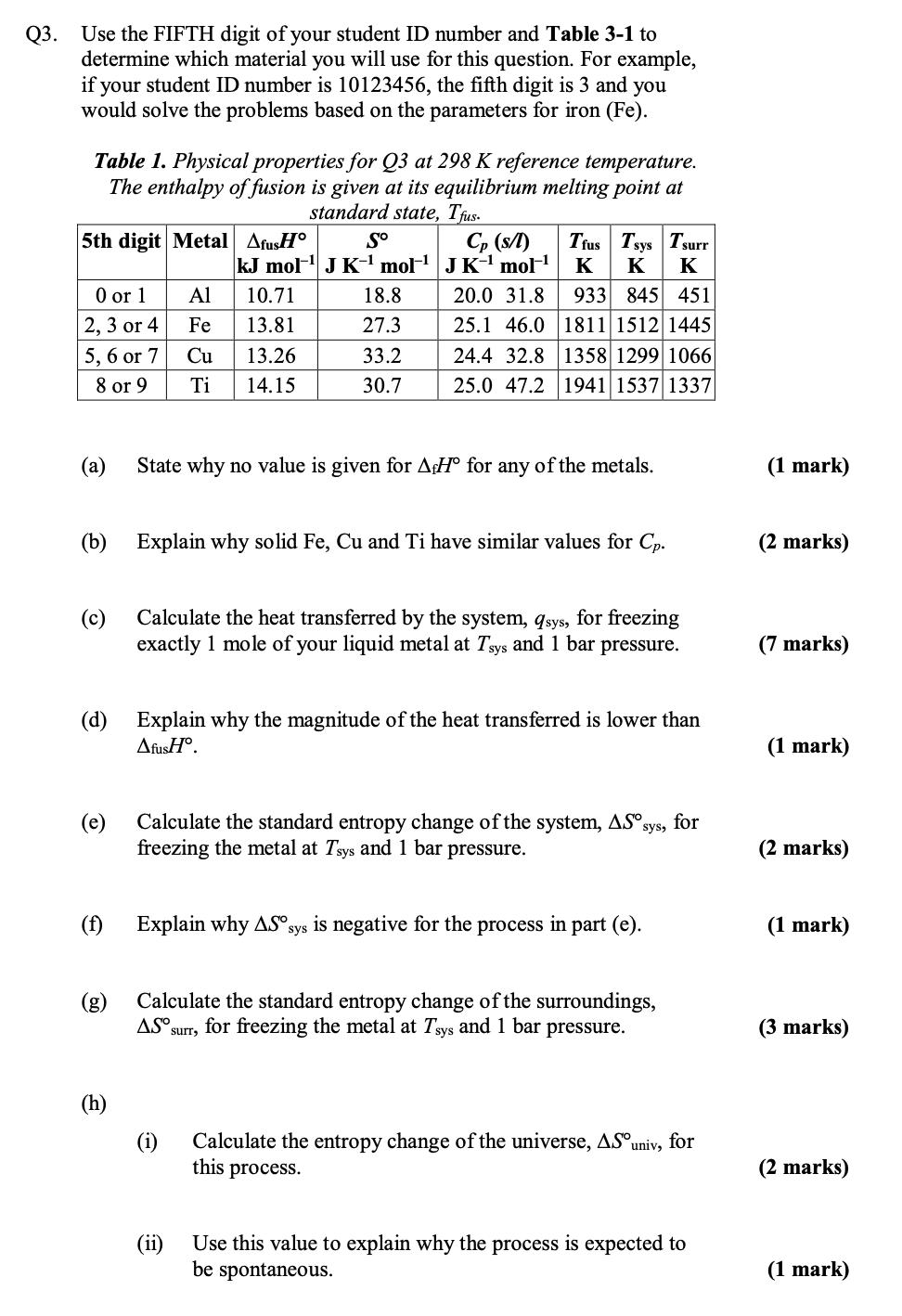
Q3. Use the FIFTH digit of your student ID number and Table 3-1 to determine which material you will use for this question. For example, if your student ID number is 10123456, the fifth digit is 3 and you would solve the problems based on the parameters for iron (Fe). Table 1. Physical properties for Q3 at 298 K reference temperature. The enthalpy of fusion is given at its equilibrium melting point at standard state, Th Trus Tsys Tsurr 5th digit Metal AfusH So kJ mol JK' mol C (n JK mol K K K 0 or 1 Al 10.71 18.8 20.0 31.8 933 845 451 2, 3 or 4 Fe 13.81 27.3 25.1 46.0 1811 1512 1445 5, 6 or 7 Cu 13.26 33.2 24.4 32.8 1358 1299 1066 8 or 9 Ti 14.15 30.7 25.0 47.2 1941 1537 1337 (a) State why no value is given for AH for any of the metals. (1 mark) (b) Explain why solid Fe, Cu and Ti have similar values for Cp. (2 marks) (c) Calculate the heat transferred by the system, qsys, for freezing exactly 1 mole of your liquid metal at Tys and 1 bar pressure. (7 marks) (d) Explain why the magnitude of the heat transferred is lower than AfusH. (1 mark) (e) Calculate the standard entropy change of the system, AS sys for freezing the metal at Tays and 1 bar pressure. (2 marks) (f) Explain why ASO sys is negative for the process in part (e). (1 mark) (g) Calculate the standard entropy change of the surroundings, AS surr, for freezing the metal at Tys and 1 bar pressure. (3 marks) (h) (i) Calculate the entropy change of the universe, ASO univ, for this process. (2 marks) (ii) Use this value to explain why the process is expected to be spontaneous. (1 mark)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


