Answered step by step
Verified Expert Solution
Question
1 Approved Answer
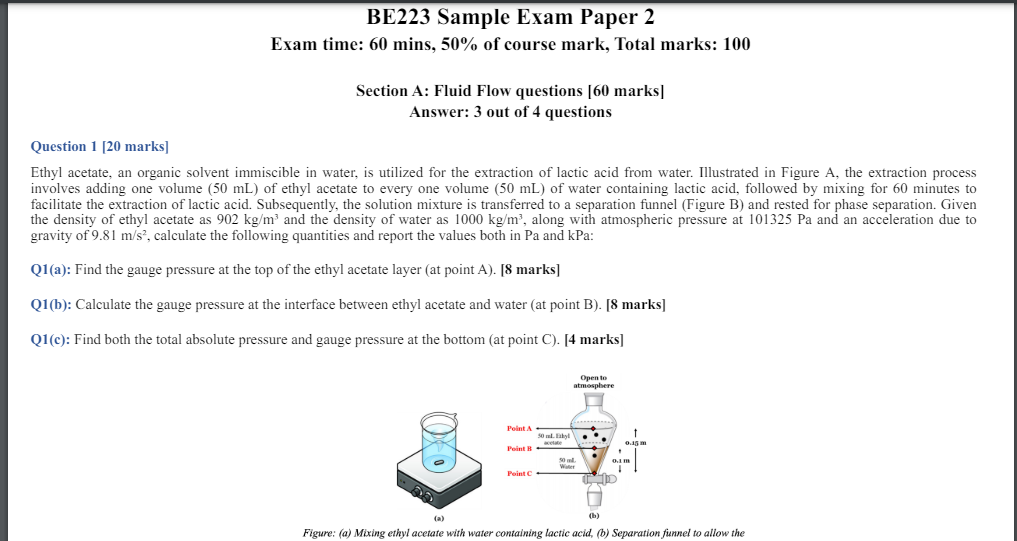
Question 1 [ 2 0 marks ] Ethyl acetate, an organic solvent immiscible in water, is utilized for the extraction of lactic acid from water.
Question marks
Ethyl acetate, an organic solvent immiscible in water, is utilized for the extraction of lactic acid from water. Illustrated in Figure A the extraction process
involves adding one volume of ethyl acetate to every one volume of water containing lactic acid, followed by mixing for minutes to
facilitate the extraction of lactic acid. Subsequently, the solution mixture is transferred to a separation funnel Figure B and rested for phase separation. Given
the density of ethyl acetate as and the density of water as along with atmospheric pressure at and an acceleration due to
gravity of calculate the following quantities and report the values both in and kPa :
Qa: Find the gauge pressure at the top of the ethyl acetate layer at point A marks
Qb: Calculate the gauge pressure at the interface between ethyl acetate and water at point B marks
Qc: Find both the total absolute pressure and gauge pressure at the bottom at point C marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


