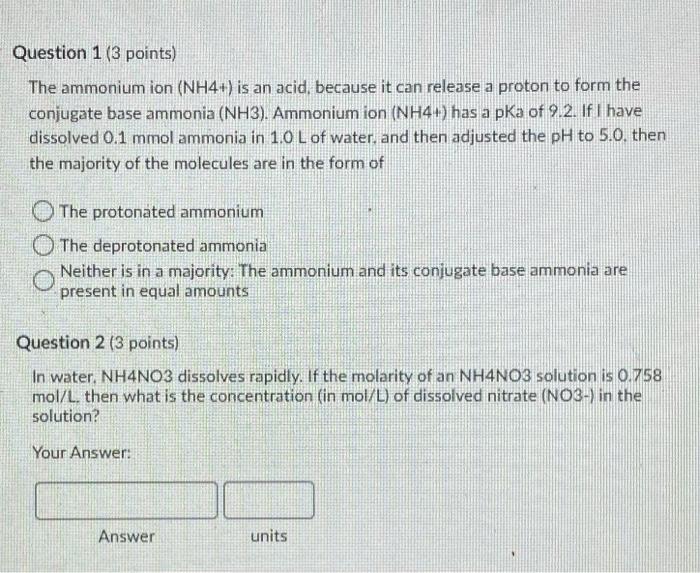
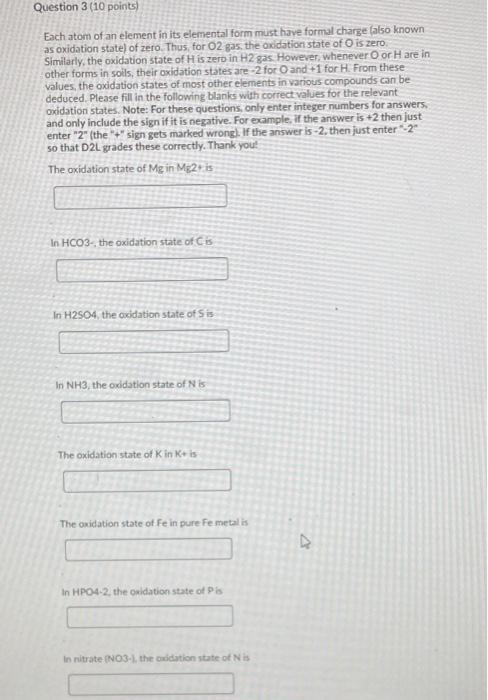
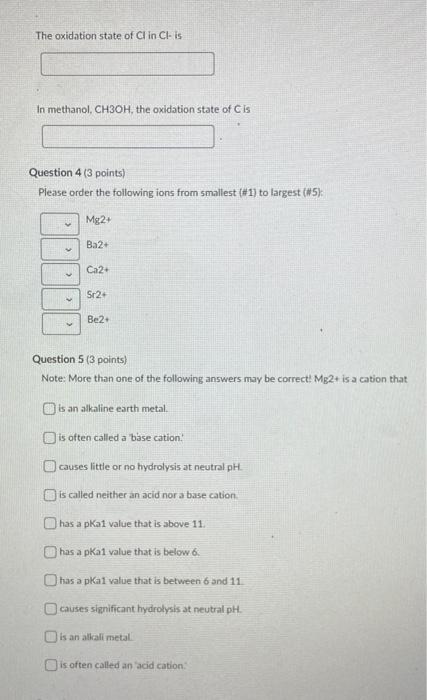
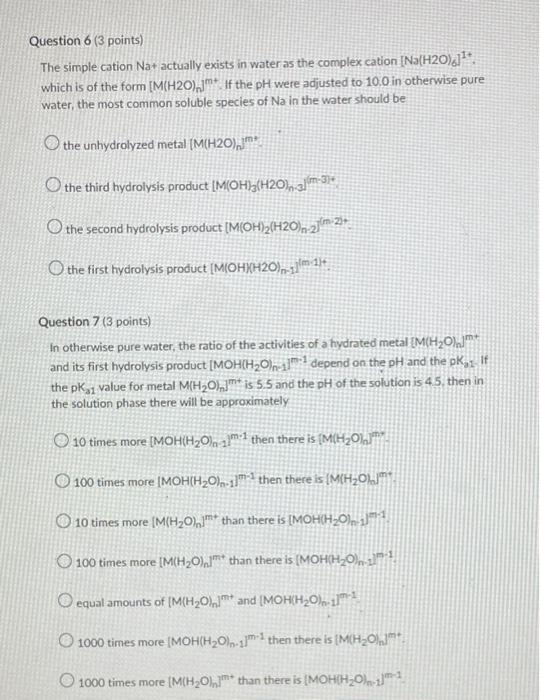
Question 1 (3 points) The ammonium ion (NH4+) is an acid, because it can release a proton to form the conjugate base ammonia (NH3). Ammonium ion (NH4+) has a pka of 9.2. If I have dissolved 0.1 mmol ammonia in 1.0 L of water, and then adjusted the pH to 5.0. then the majority of the molecules are in the form of The protonated ammonium The deprotonated ammonia Neither is in a majority: The ammonium and its conjugate base ammonia are present in equal amounts Question 2 (3 points) In water, NH4NO3 dissolves rapidly. If the molarity of an NH4NO3 solution is 0.758 mol/L, then what is the concentration (in mol/L) of dissolved nitrate (NO3-) in the solution? Your Answer: Answer units Question 3 (10 points) Each atom of an element in its elemental form must have formal charge (also known as oxidation state) of zero. Thus, for O2 gas, the Oxidation state of O is zero Similarly, the oxidation state of His zero in H2 gas. However, whenever Oor Hare in other forms in soils, their oxidation states are -2 for O and +1 for H. From these values, the oxidation states of most other elements in various compounds can be deduced. Please fill in the following blanks with correct values for the relevant oxidation states. Note: For these questions, only enter integer numbers for answers. and only include the sign if it is negative. For example, if the answer is +2 then just enter "2" (the "+" sign gets marked wrong). If the answer is-2. then just enter 2" so that D2L grades these correctly. Thank you! The oxidation state of Me in Mg2 is In HCO3- the oxidation state of Cis In H2SO4, the oxidation state of Sis In NH3, the oxidation state of Nis The oxidation state of Kin Kis The oxidation state of Fe in pure Fe metalls In HPO4-2. the Oxidation state of Pis In nitrate NO3-. the baddation state of Nis The oxidation state of Clin CH IS In methanol, CH3OH, the oxidation state of Cis Question 4 (3 points) Please order the following ions from smallest (#1) to largest (#5) Mg2+ Ba2+ Ca2 Sr2- Be2 Question 5 (3 points) Note: More than one of the following answers may be correct! Mg2+ is a cation that is an alkaline earth metal. is often led a base cation: causes little or no hydrolysis at neutral pH. is called neither an acid nor a base cation. has a pkai value that is above 11. has a pKal value that is below 6. has a pkai value that is between 6 and 11 causes significant hydrolysis at neutral pH. is an alkali metal is often called an acid cation: Question 6 (3 points) The simple cation Na+ actually exists in water as the complex cation Na(H20).j1*. which is of the form [M(H20), If the pH were adjusted to 100 in otherwise pure water, the most common soluble species of Na in the water should be the unhydrolyzed metal (M(H20),jm O the third hydrolysis product |M(OH)3(H20), 31m-33 O the second hydrolysis product (M(OH)2(H20)n-2](m 27. O the first hydrolysis product (MOHXH2O) n.1m 14 Question 7 (3 points) In otherwise pure water, the ratio of the activities of a hydrated metal (M(H20) and its first hydrolysis product (MOHCH20 r11:1 depend on the pH and the pK, If the pk 1 value for metal M(H20), is 5.5 and the pH of the solution is 45 then in the solution phase there will be approximately 10 times more (MOH(H20) 2:1 then there is (M(H20), O 100 times more (MOH(H20)n-1171 then there is (M(H2OLJm 10 times more (M(H20).jm* than there is [MOH(H20):191 O 100 times more [M(H20),1m* than there is (MOH(H2O), 221 O equal amounts of [M(H20),]** and (MOH(H2O), 1171 1000 times more (MOH(H2O), then there is IMHO 1000 times more MH2O1,7m than there is IMOHH20).- 11 Question 8 (2 points) If equal aqueous concentrations of dilute Mg2+ and Cu2+ were sprayed onto soil rich in iron oxides, then Mg? would be adsorbed more strongly to the oxide minerals than Cu2+ True False Question 9 (2 points) If equal aqueous concentrations of dilute Pb2+ and Ca2+ were sprayed onto soil rich in iron oxides, then Pb2+ would be adsorbed more strongly to the oxide minerals than Ca 2+ True False Question 10 (3 points) A soil test report tells you that your sample has a soil organic carbon (SOC) content of 0.051 g SOC/g soil. Our class notes on organic matter give a simple method for converting between SOC and soil organic matter (SOM) content. What is the approximate SOM content (in g SOM/g soil, to the nearest 0.0001 g SOM/g soil) of the sample described in the soil test report? Your Answer Answer units Question 11 (5 points) Fluazitop-p-butyl (R)2-14445I-(trifluoro-methyo-2-pyridinyloxyl-phenoxy! propanoate) is a common phenoxy herbicide, used widely for postemergence control of annual and perennial grass weeds, it is used on soybeans and other broad-leaved crops such as carrots, spinach, potatoes, and ornamentals. It is sold under the brand name Fusilade (trademark of Syngenta). The solubility of this pesticide in water is about 5 mg/L and the sorption of the pesticide to soil organic matter is characterized by an organic-carbon-normalized partitioning coefficient of 5.000, Suppose the soil on a sod farm contains 2.51% soil organic carbon, fluazitop-p-buty is applied to grass at the soil surface, and then it rains. The infiltrating rainwater contains an average pesticide concentration of 5 mg pesticidel. If we assume that sorption of this pesticide to soil is dominated by linear partitioning into soil organic matter, then please estimate the sorption of this pesticide in me pesticide/kg soil) Your Answer Answer units